POC System Delivers Lab-Quality PCR Results Directly from Swab Sample in Minutes
Posted on 09 Jun 2025
Medical and laboratory staff in primary care settings are multi-skilled and routinely multitask to deliver care with the speed and convenience expected of modern medicine. This places a strong requirement for ease-of-use on the tools they utilize. To address this requirement, a new ultra-fast point-of-care molecular diagnostics platform delivers highly sensitive PCR results for key respiratory pathogens directly from a swab sample in less than ten minutes.
The VELO system from LEX Diagnostics (Cambridgeshire, UK) uses a proprietary cartridge-based design that eliminates the need for external liquid handling, providing ease of use and reliability. The system supports multiplex testing for key respiratory pathogens, including Influenza A, Influenza B, and COVID-19, and delivers PCR results in six to ten minutes. The VELO system can be easily integrated into clinical workflows across primary care settings, urgent care clinics, pharmacies, physician office laboratories, and decentralized acute settings.
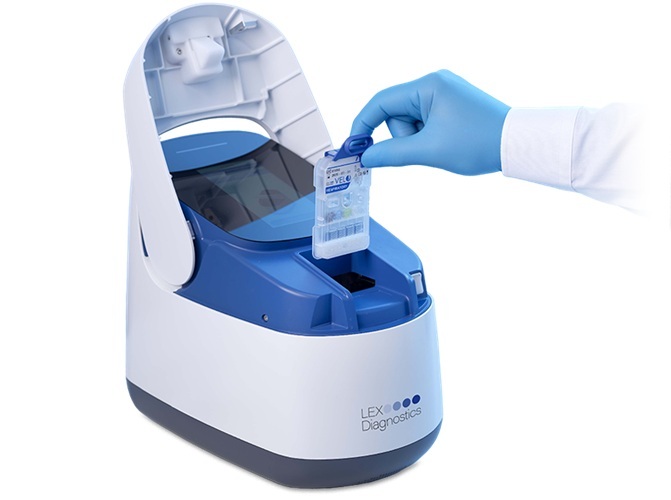
LEX has completed clinical studies of the VELO system and the Influenza/Covid assay in the U.S. during the 2024/2025 respiratory season. The company has submitted dual applications to the U.S. Food and Drug Administration (FDA) for 510(k) clearance and CLIA waived status for its VELO system. It expects U.S. market clearance in late 2025 or early 2026, which is in line with the anticipated FDA review timelines.
“We believe the LEX VELO system will redefine point-of-care testing by equipping healthcare providers with lab-quality results in minutes, enabling faster clinical decisions and improved patient outcomes without compromising quality while improving workflow—an achievement that will set us apart in the marketplace,” said Ed Farrell, Chief Executive Officer of LEX Diagnostics.
Related Links:
LEX Diagnostics














