Ultrasensitive Test Detects and Serially Monitors Intact Virus Levels in COVID-19 Patients
Posted on 24 Jan 2025
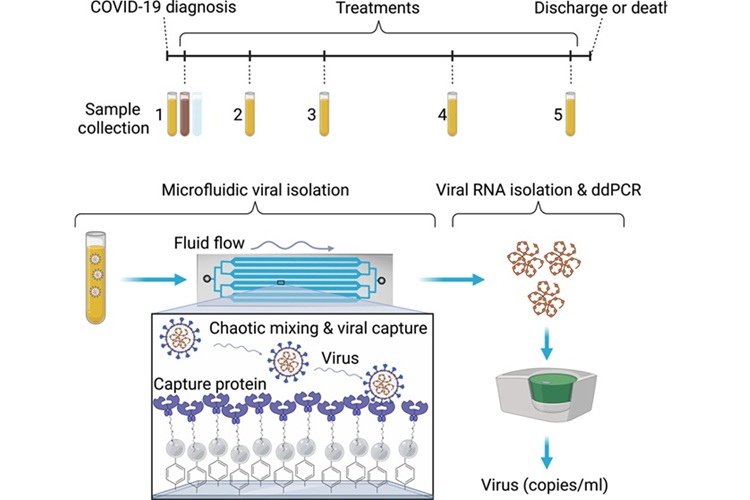
The ability to isolate and detect whole viruses from complex biofluids could enhance our understanding of how severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as well as other viral infections, spreads within the host, providing valuable insights into their dynamics. Quantifying whole viral particles could inform infectivity and reveal a potential link between viral load and organ damage. Researchers have now found that a method originally created for cancer detection can also identify and track even trace amounts of intact SARS-CoV-2 viral particles in blood and other fluids from patients with acute COVID-19 infections, offering promise for improving future treatment strategies.
In the early days of the pandemic, scientists at Mass General Brigham (Somerville, MA, USA) sought to adapt their cancer vesicle isolation technique to detect SARS-CoV-2 in biofluids like blood, stool, and saliva. They quickly assembled a multidisciplinary team to adapt their technology and expand the potential for detecting intact viruses. Their research, published in Science Advances, demonstrated that this method could detect as few as three viral particles in 1 milliliter of blood. When applied to more than 150 samples (103 plasma, 36 saliva, and 29 stool samples) from COVID-19 patients, the technique accurately measured viral levels over time, with intact viral particles detectable up to 50 days after the initial infection.
“With clinical needs changing, the ability to serially monitor viral load in this manner has great potential for guiding the treatment of patients with long Covid,” said co–senior author Shannon L. Stott, PhD. “This versatile technology could also have widespread applications in viral monitoring for current and future infectious diseases.”













