Neonatal Saliva Enables Ultrasensitive Detection of Sepsis-Associated Proteins
Posted on 21 Jan 2025
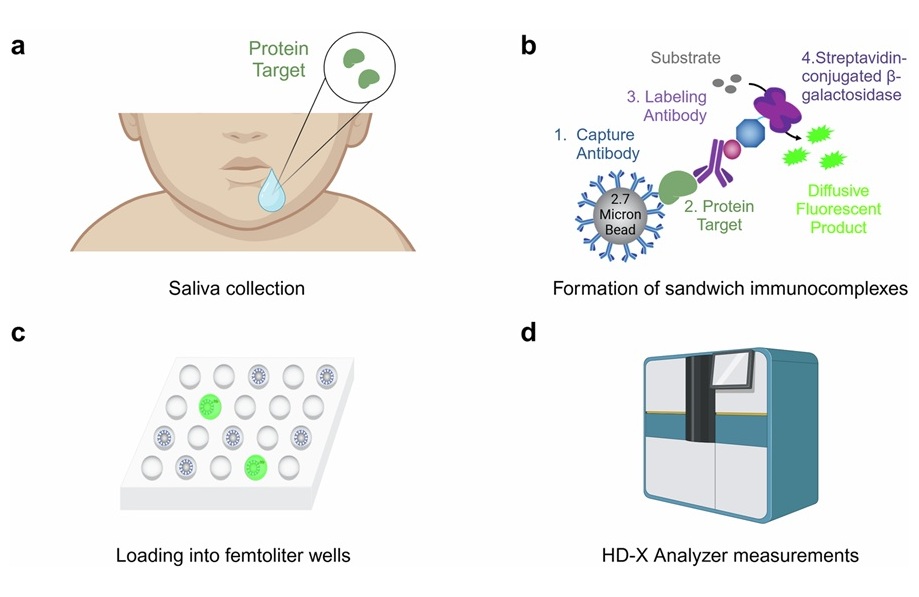
Sepsis is a critical condition that affects millions of newborns each year. Current diagnostic procedures involve painful, repetitive blood collections to isolate and culture the microorganism responsible for the infection. Results can take up to five days to return, potentially exposing newborns to unnecessary antibiotic treatments. Developing a quicker, non-invasive method to assess infectious status based on the host's immune response offers a promising alternative for infection screening. However, additional blood collection to multiplex inflammatory biomarkers in neonates remains impractical. Saliva, though difficult to work with due to much lower protein concentrations (100 to 1000 times less than blood), could provide an alternative biofluid for testing. To overcome this challenge, researchers have developed a set of six ultra-sensitive single-molecule array (Simoa) assays that can detect inflammatory biomarkers linked to neonatal sepsis even in small amounts of saliva.
This panel of six Simoa assays was developed through a collaborative effort between investigators at the University of California, San Francisco (UCSF, San Francisco, CA, USA). The inflammatory biomarkers associated with neonatal sepsis include serum amyloid A1 (SAA1), lipopolysaccharide-binding protein (LBP), chemokines CCL20, CXCL6, CXCL12, and the adipokine resistin. Using the Simoa platform, the research team demonstrated that these biomarkers could be detected in neonatal saliva. The study showed that salivary biomarkers CCL20 and CXCL6 were significantly elevated in neonates with infections and sepsis compared to uninfected ones, indicating their potential for use in neonatal infection screening.
The study, published in npj Biosensing, is the first to show that chemokines, adipokines, and acute phase reactants can be measured in neonatal saliva. The small saliva volumes (~10 μL) and low concentrations of these biomarkers (pg/mL) highlight the need for ultra-sensitive assays in developing non-invasive diagnostics. This work reinforces the value of neonatal saliva as a viable sample for infection monitoring. The researchers believe that these assays could be utilized in future diagnostic tests to distinguish between infected and uninfected neonates. Advancing salivary-based diagnostics for neonatal conditions is a significant step toward reducing the risks associated with blood draws and improving care for this vulnerable group. While these assays represent a crucial first step for diagnosing neonatal sepsis, they will require further validation to ensure consistency and reliability.













