Portable PCR Platform to Detect Multiple Pathogenic Bacteria Targets and Antibiotic Susceptibility at POC
Posted on 23 Jul 2024
Gonorrhea, the second most reported bacterial sexually transmitted infection (STI), affected approximately 82 million people globally in 2020. The incidence is higher in low and middle-income countries where the burden of drug resistance is most pronounced. Gonorrhea can lead to severe health complications such as pelvic inflammatory disease, chronic pelvic pain, and infertility. Since not all patients show symptoms, the true burden of the disease is likely much greater than reported cases suggest. To combat this, rapid and advanced diagnostics are crucial for detecting drug-resistant bacterial infections early and guiding effective treatment. Now, a portable PCR platform with high multiplexing capabilities is being developed to identify multiple pathogenic bacterial targets and their antibiotic susceptibility, thus aiding in point-of-care treatment decisions.
Prompt Diagnostics (Baltimore, MD, USA) has been awarded USD 1 million by the Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X, Boston, MA, USA) to further the development of its portable PCR platform. Prompt's innovative platform is designed to be low-cost and portable, capable of delivering laboratory-quality diagnostics that can inform treatment choices directly at the clinical site. The platform's advanced PCR assays can quickly provide results, allowing healthcare providers to administer targeted therapies during a patient's initial visit. The tests efficiently miniaturize and automate the processes of sample extraction and the amplification of DNA and RNA targets into economical cartridges. The high multiplexing abilities of the Prompt platform enable the detection of multiple diseases simultaneously and the genotyping of various mutations to assess variants and predict antibiotic resistance amidst evolving pathogens.
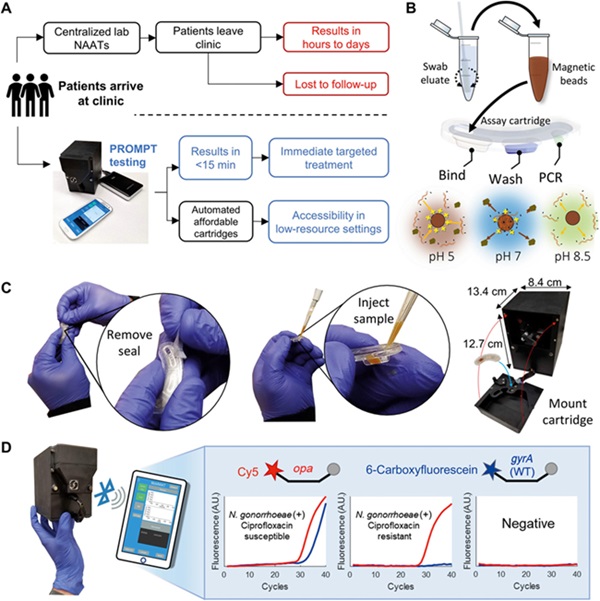
The funding from CARB-X will help Prompt further develop its technology, particularly to demonstrate its capability in detecting gonorrhea and accurately determining drug resistance through genetic mutations, including those that confer resistance to third-generation cephalosporins. The technology's sophisticated melt-curve analysis could expand the options for reliable antibiotic susceptibility testing. If successful, Prompt's approach could significantly improve treatment management and foster antimicrobial stewardship, helping to curb the spread and impact of multi-drug resistant gonorrhea worldwide, especially in areas where access to comprehensive healthcare is limited.
“We are very excited to work with CARB-X to push our Prompt diagnostic platform to market,” said Alexander Trick, PhD, Co-Founder and CEO of Prompt Diagnostics. “In addition to funding, the expertise of the CARB-X team and collaborators will provide critical insight and guidance for product development and maximizing clinical impact.”
“Prompt’s technology has the potential to efficiently detect the presence of ceftriaxone-resistant gonorrhea, which poses a growing and significant global public health risk,” added Erin Duffy, PhD, R&D Chief of CARB-X. “Rapid, sophisticated diagnostics are essential to addressing drug-resistant bacterial infections globally, as they detect infections as early as possible while also directing healthcare providers to the most appropriate treatment. We are enthusiastically partnering with Prompt to better understand the capabilities of their PCR platform and how it could support decentralized rapid ID and susceptibility testing for all of the major classes of antibiotics.”
Related Lin
Prompt Diagnostics
CARB-X














