Simple Saliva DNA Test Identifies Prostate Cancer Patients at Risk for Radiation Toxicity
Posted on 09 Jul 2024
Radiation therapy is the standard treatment for prostate cancer, but it can lead to long-lasting side effects like urinary and bowel complications, significantly affecting patients' quality of life. Globally, men with prostate cancer endure the highest number of years living with disabilities resulting from treatment side effects, underscoring both the high prevalence of the disease and the effectiveness of modern therapy in achieving high cure rates. Now, researchers are further advancing their work in personalizing cancer treatment to enhance the quality of life for prostate cancer patients by predicting and preventing severe long-term side effects caused by radiation therapy.
Investigators at the UCLA Health Jonsson Comprehensive Cancer Center (Los Angeles, CA, USA) have received a USD 1.8 million grant from the National Cancer Institute (NCI, Bethesda, MD, USA) to validate and leverage biomarkers that predict adverse side effects from radiation therapy. Their study targets specific genetic markers known as mirSNPs, which are promising in predicting side effects from cancer treatments. A critical biomarker identified by the team, PROSTOX, has been shown to predict late genitourinary toxicity following advanced radiation therapy. PROSTOX is effective in predicting toxicity from two radiation therapy types: Stereotactic body radiation therapy (SBRT), which administers radiation in high doses over 5-7 sessions, and conventionally fractionated radiation therapy (CFRT), involving 35-45 sessions.
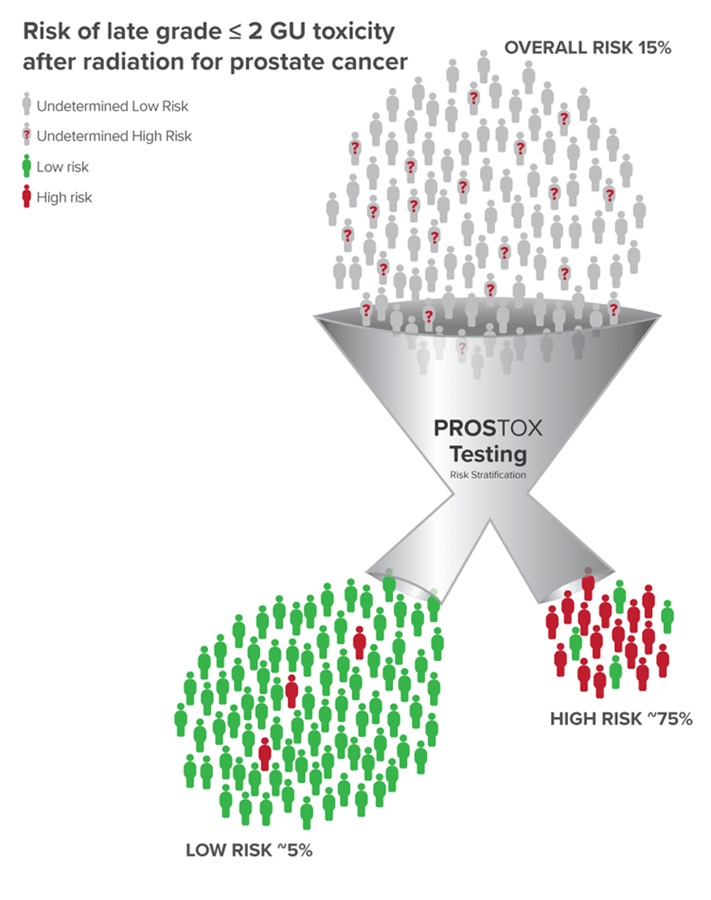
This test enables the assessment of whether a patient is at low or high risk for late genitourinary toxicity following SBRT or CFRT. Understanding the risk level can assist patients and physicians in selecting the most appropriate and safest treatment strategy. The researchers aim to further validate the predictive accuracy of PROSTOX in more patient groups. Additionally, they plan to explore the biological variations among patients with these biomarkers and assess the potential of highly targeted adaptive radiotherapy to minimize these and other side effects for all patients.
“By understanding the mirSNP genetic markers that predispose patients to adverse side effects from cancer therapy we can tailor these therapies to minimize harm and maximize efficacy,” said co-investigator Dr. Joanne Weidhaas, professor of radiation oncology, vice chair and head of translational research and co-developer of the PROSTOX test. “This work has the potential to improve the lives of countless patients.”
Related Links:
UCLA Health Jonsson Comprehensive Cancer Center
MiraDx
NCI










 Analyzer.jpg)



