Groundbreaking Molecular Diagnostic Kit to Provide Lyme Disease Detection in Minutes
Posted on 31 May 2024
Lyme disease, transmitted through tick bites, is a bacteria-caused illness that impacts 1.2 million individuals annually. The standard methods for diagnosing this disease include clinical examinations, visual evaluations, or immunological tests, all of which depend on the presence of advanced symptoms that might take months or even years to appear. Consequently, many cases remain untreated and undiagnosed early on, complicating treatment and leading to the severe, long-term impacts of Lyme disease. Now, a groundbreaking patented molecular diagnostic kit is poised to provide game-changing autonomous detection of Lyme disease within minutes.
Presently, Lyme disease testing methods aim to identify antibodies produced by the body in reaction to the infection. However, since the development of these antibodies can take several weeks, tests performed soon after infection often yield false negatives. En Carta Diagnostics (Paris, France) has developed a molecular diagnostic platform that effectively addresses this issue. This platform, which utilizes a simple cassette format, provides rapid, point-of-care diagnostics with remarkable accuracy. The underlying technology has been improved over a decade of extensive research and employs aptamers—molecules engineered for their strong binding abilities and high specificity to their target. These aptamers can be customized to detect a wide array of genetic, pathogenic, or veterinary markers, significantly broadening potential applications.
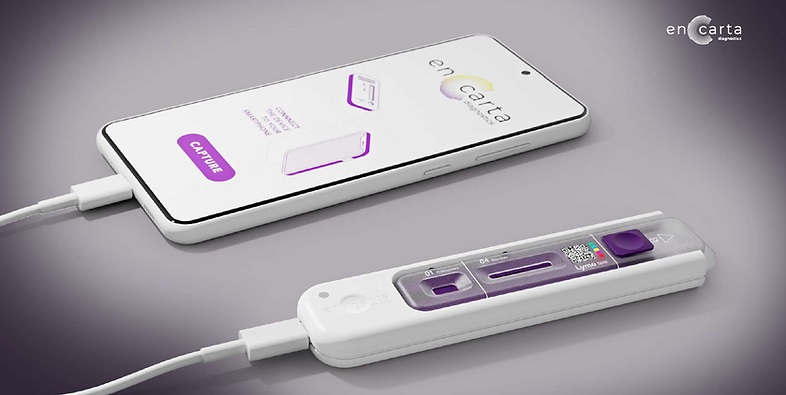
En Carta’s diagnostic technology, which drives their detection kits, has undergone rigorous validation against multiple pathogens and has been tested with real patient samples. The company is now developing a diagnostic kit specifically for Lyme disease that promises to deliver results autonomously in mere minutes. The portable and compact cassette enables test performance quickly and easily by anyone and anywhere. Powered by a smartphone, a user friendly and intuitive app allows for easy result interpretation and integration with healthcare systems. By enabling molecular PCR-grade detection of Lyme bacteria right at the tick bite, the test will eliminate medical wandering often experienced by Lyme patients. In addressing Lyme disease as its first application, the company is responding to the growing urgency of this disease, which is now the most common vector-borne illness in the Northern Hemisphere, affecting 1.2 million people each year.
"Today, we have a very robust platform technology leveraged for accuracy, speed and cost, which are key in the Point-of-Care space,” said Guillaume Horreard, CEO at En Carta. “As a first step, we are focusing on Lyme disease, where the sizeable patient population, accelerated growth of the problem and need for rapid diagnosis following a tick bite make our product indispensable.”
Related Links:
En Carta Diagnostics














