10 Minute RT-PCR Point-of-Care System Detects Up To 32 Targets Per Sample
Posted on 21 Mar 2024
A 10-minute RT-PCR point-of-care (POC) system and respiratory panel are set to change the discussion on next-generation POC testing.
Autonomous Medical Devices Incorporated (AMDI, Santa Ana, CA, USA) is developing its Fast PCR System, a CLIA-waivable RT-PCR POC system that can detect up to 32 targets per sample in less than 10 minutes. The AMDI Fast PCR system is a compact, easy-to-use molecular point-of-care system that utilizes multiplexing capability specifically for CLIA-waived environments. It can run up to 32 targets at a time on a single sample in <10 minutes using a disposable disc without the need for a separate, offline sample preparation. At the core of the AMDI ultrafast system is the company’s Hyperbaric Heating (HBH) sample prep technology combined with its ultrafast PCR chemistry. The HBH technology eliminates the time constraints and costs associated with traditional sample preparation.
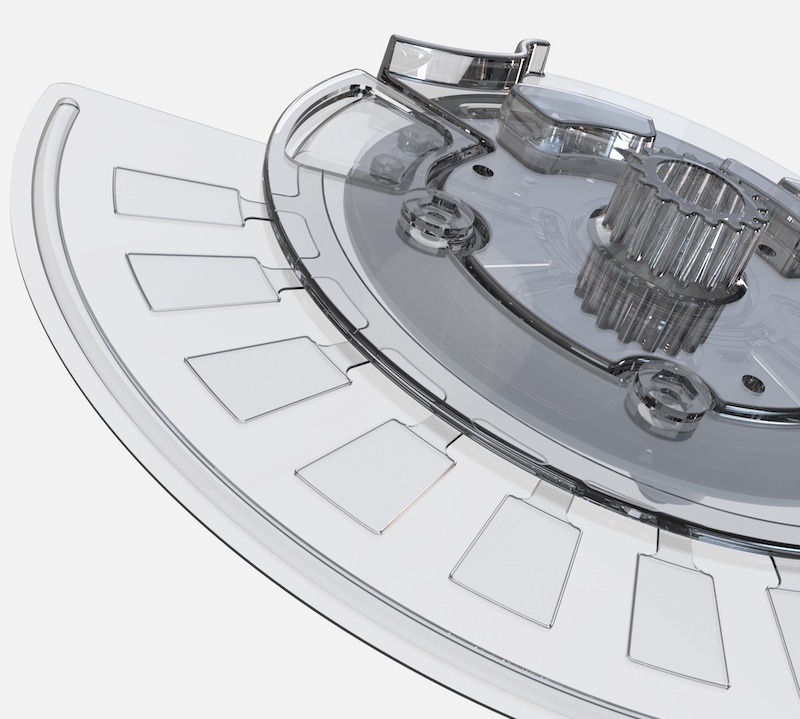
Combining HBH with AMDI’s ultrafast thermal cycling allows the test time to stay under 10 minutes regardless of the number of targets put on the test disc. The AMDI ultrafast system is designed to deliver highly sensitive, lab-quality results with the accessibility needed to address the testing needs of both the current and future pandemics. AMDI is also developing the first test panel for the Fast PCR system, targeting the detection of multiple respiratory viruses. The AMDI Respiratory Panel tests for the four most probable viruses that cause respiratory tract infections since the pandemic, including influenza A and B, respiratory syncytial virus, and SARS-CoV-2.
"Our market research indicates that our technology, when combined with cloud connectivity, can revolutionize patient care in the CLIA Waived setting," said David Okrongly, CEO of AMDI.
Related Links:
AMDI