Ultra-Portable POC Molecular Diagnostics System to Instantly Detect Infectious Diseases
Posted on 02 Feb 2024
Infectious diseases pose a significant threat to human health and have a substantial economic impact globally. Timely and accurate identification of pathogens is key for prompt treatment, improved patient outcomes, and halting the spread of diseases. Traditional pathogen detection methods are usually conducted in well-equipped clinical labs, relying on advanced equipment and specialized staff. Furthermore, pre-analytical steps such as lengthy transportation can skew results and extend turnaround times (TAT), hindering effective control of infectious diseases, especially in areas with limited resources. Now, a rapid RT-PCR platform being developed to provide highly accurate, rapid, and affordable diagnoses of infectious diseases at point-of-care and near-patient settings could enable immediate treatment initiation and curtail potential disease transmission.
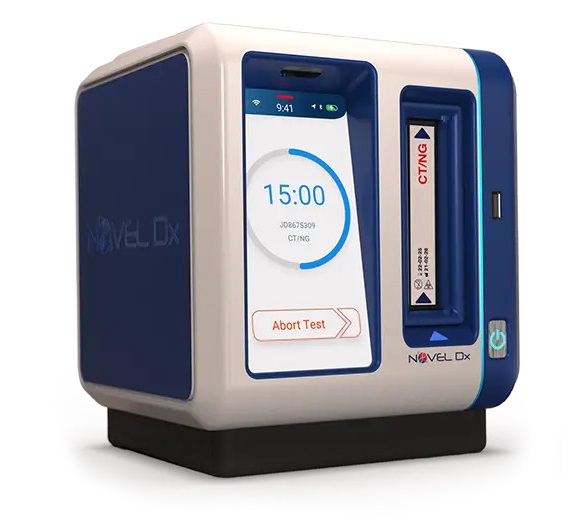
Novel Microdevices (Baltimore, MD, US) is advancing its affordable, portable, rapid, multiplex, and user-friendly PCR testing solution for infectious diseases. This innovative technology simplifies complex scientific processes, typically carried out in high-complexity labs, into a compact device. Point-of-care diagnostics offered by the Novel Dx device allow for immediate results, enabling prompt action instead of the extended waiting times associated with traditional laboratory tests. The Novel Dx device employs Nucleic Acid Amplification Techniques (NAAT), which is the same method used in clinical reference laboratories, to detect the genetic material of pathogens.
The Novel Dx molecular testing platform utilizes polymerase chain reaction (PCR) technology in a disposable cartridge. The patient's sample is added to this cartridge, which is then inserted into the Novel Dx system. The system analyzes genetic sequences to identify diseases and detect genetic mutations that may make bacteria resistant to antibiotics. Novel Dx is capable of providing lab-quality results in less than 15 minutes, allowing patients to be tested, diagnosed, and treated all within a single visit, thus eliminating the wait for results from more complex labs. Designed for portability and weighing just four pounds, the Novel Dx diagnostic tool is battery-operated, making it especially suitable for use in low-resource settings and low-and middle-income countries (LMICs).














