Cell-Based Assay Detects Nodal/Paranodal Autoantibodies in Novel Type of Neuropathy
Posted on 26 Jan 2024
The four autoantibodies against the nodal/paranodal proteins neurofascin 186 (NF186), neurofascin 155 (NF155), contactin-1 (CNTN1), and contactin-associated protein-1 (CASPR1) are recognized as biomarkers of a unique type of inflammatory neuropathy, termed autoimmune nodopathies. These diseases are clinically similar to Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy (CIDP) but are pathologically distinct. The target antigens NF186, NF155, CNTN1, and CASPR1 are membrane proteins situated in or around the nodes of Ranvier — gaps in the myelin sheath that enable fast conduction of nerve signals. The autoantibodies are considered pathogenic, and the resulting immune reactions result in slowed conduction or even complete failure of impulse transmission. Autoimmune nodopathies manifest as acute, subacute, or chronic onset sensory-motor neuropathies with distinct clinical phenotypes. They have an aggressive onset but good long-term prognosis when treated with immunosuppressives.
Of the four antibodies, anti-NF155 is the most common finding in autoimmune nodopathies. Patients with these antibodies have predominantly distal motor weakness, sensory disturbances, ataxia, and tremors. Anti-NF186 antibodies are associated with a severe subacute onset polyradiculoneuropathy phenotype, but without tremors. Patients with pan neurofascin autoimmunity have a fulminant disease course and frequently require mechanical ventilation. Antibodies to CNTN1 are associated with sensory ataxia and distal motor weakness, while neuropathy with anti-CASPR1 is characterized by sensory ataxia, motor weakness, neuropathic pain, opthalmoparesis, facial paralysis, and respiratory failure.
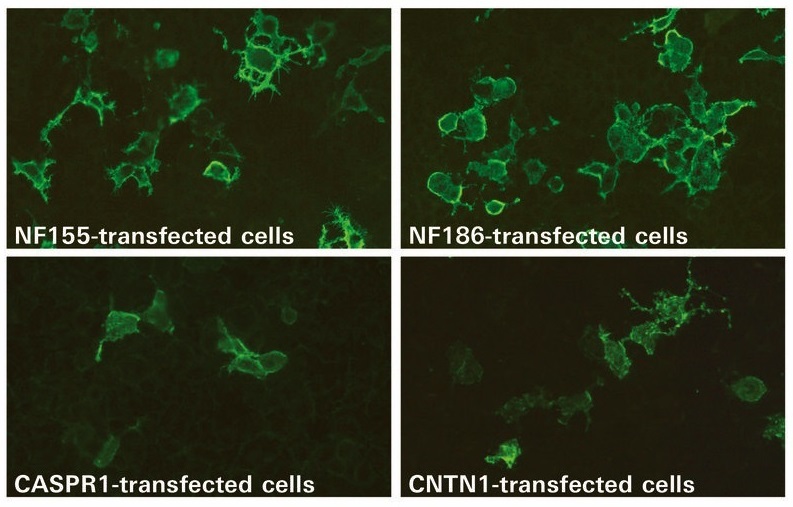
Now, a new cell-based assay (CBA) from EUROIMMUN (Lübeck, Germany) provides multiparameter detection of the four autoantibodies against the nodal/paranodal proteins and is based on transfected human cell lines expressing each target antigen. This CBA technology enables authentic presentation of the fragile conformation-dependent antigens, allowing highly sensitive antibody detection. The four antibodies are determined simultaneously using a BIOCHIP Mosaic composed of miniature cell substrates that are incubated together with one patient sample. The analysis procedure is simple and can be performed in any laboratory familiar with indirect immunofluorescence. Automation options are available to facilitate the processing and evaluation. The new BIOCHIP Mosaic for the detection of antibodies against NF186, NF155, CNTN1, and CASPR1 is currently available for research use only. The new parameters expand EUROIMMUN’s unparalleled portfolio of assays for the detection of neural autoantibodies, which comprises over 60 autoantibody specificities, including many novel and exclusive parameters.
Related Links:
EUROIMMUN














