Autoantibody Status Guides Treatment in Myasthenia Gravis
Posted on 20 Dec 2023
Autoantibody status plays a significant role in diagnosis and therapy decisions for myasthenia gravis (MG), an immune-mediated neuromuscular junction disorder. The key serological markers in MG are antibodies against nicotinic acetylcholine receptors (AChR), which are found in about 85% of cases, and antibodies against muscle-specific kinase (MuSK), which are observed in approximately 6% of cases, the majority of which are anti-AChR negative. 15% of MG cases are seronegative. The antibody-mediated processes in MG result in the loss of functional AChR at the motor endplate, either through direct blocking, antigen modulation, or complement-mediated damage of the muscle membranes. Anti-AChR antibodies belong to the IgG1 subclass and can activate the complement cascade. In AChR antibody-positive cases, complement-inhibiting drugs are among the recommended therapies. On the other hand, anti-MuSK antibodies belong to the non-complement binding IgG4 subclass, as a result of which complement inhibitors are ineffective for this form. Hence, therapy selection should consider the autoantibody status along with disease activity, thymus pathology, and the patient’s age.
Currently, the gold standard for detection of anti-AChR and MuSK antibodies is radioimmunoprecipitation assay (RIPA) although it comes with a disadvantage of the need for radioactive reagents. Cell-based indirect immunofluorescence assays (CBAs) offer a viable alternative that is easier to implement. EUROIMMUN (Lübeck, Germany) has developed CBAs based on transfected human cell lines expressing the corresponding target antigens AChR (adult: AChR-E and fetal: AChR-G) and MuSK. In an evaluation study published in the Journal of the Neurological Sciences, the EUROIMMUN Anti-AChR CBA demonstrated similarly excellent specificity and a significantly higher sensitivity for MG compared to RIPA, detecting anti-AChR in 21% of patients otherwise considered to be seronegative.
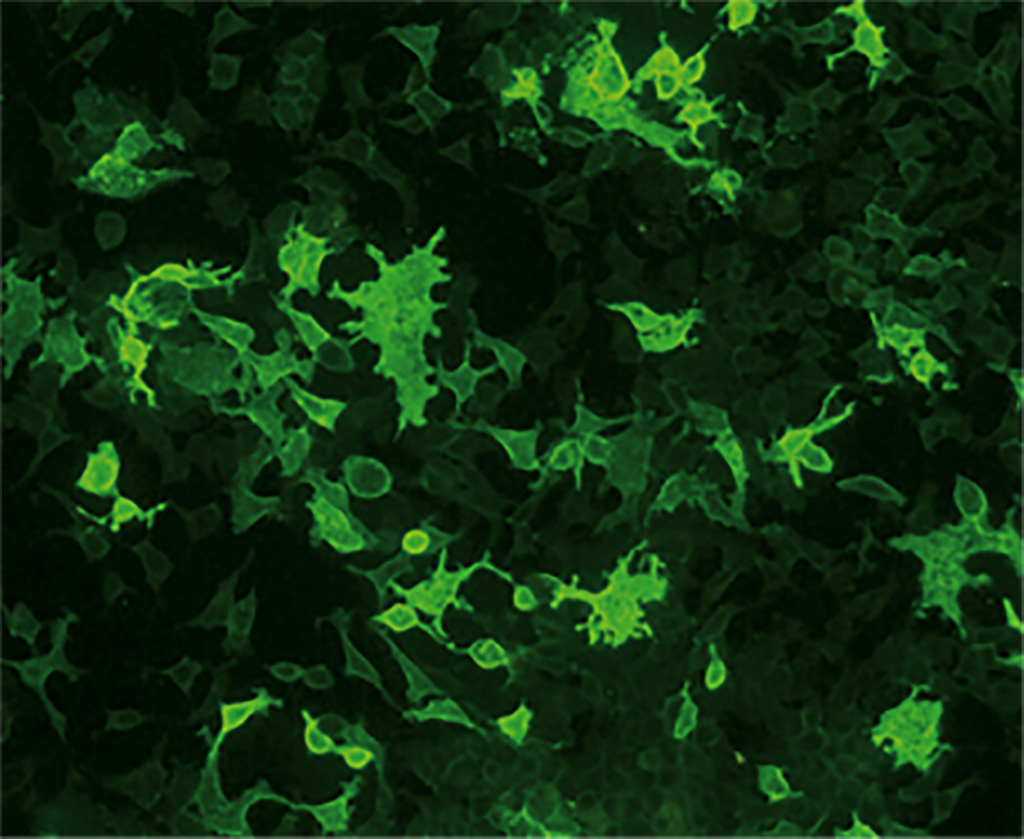
In a further analysis, the Anti-AChR and Anti-MuSK CBAs demonstrated high positive and 100% negative agreement with RIPA in patients with clinically confirmed MG. CBAs thus provide equivalent or superior performance to RIPA without the use of radioactive materials. Moreover, anti-AChR and anti-MuSK antibodies can be analyzed simultaneously using the EUROIMMUN Myasthenia gravis Mosaic 2, which provides a composite of CBAs for adult/fetal AChR and MuSK together with a control substrate. This test is currently the only commercially available multiparameter assay for anti-AChR and anti-MuSK. The CBAs are CE-marked. The CBA procedure is simple and can be performed in any laboratory familiar with indirect immunofluorescence. There are automation options available to aid the processing and evaluation, allowing for the assay’s smooth integration into laboratory routines.
Related Links:
EUROIMMUN














