Cell-Based Assays Recommended for Autoantibody Detection in Demyelinating Diseases
Posted on 05 Dec 2023
Neuromyelitis optica spectrum disorders (NMOSD) are acquired demyelinating disorders of the central nervous system (CNS) that affect the optic nerves, spinal cord and brainstem. NMOSD must be diagnostically distinguished from the more common demyelinating disease multiple sclerosis (MS), which can resemble NMOSD in the initial stages but has different therapy requirements. Autoantibodies against the CNS water-channel protein aquaporin-4 (AQP4) are pathogenic for NMOSD and represent a highly specific diagnostic biomarker. Autoantibodies against myelin oligodendrocyte glycoprotein (MOG) are a marker for MOG antibody-associated diseases (MOGAD), which have clinical and radiological overlap with NMOSD. Now, the 2023 recommendations for the diagnosis of NMOSD stipulate that cell-based assays (CBA) are the gold standard for the detection of autoantibodies against AQP4 and MOG.
AQP4-IgG is a mandatory diagnostic test for NMOSD, while MOG-IgG testing is recommended in AQP4 antibody-negative cases and for differential diagnostics. Determination of both AQP4-IgG and MOG-IgG antibodies helps to delimit the diseases from each other and from classic MS. The new recommendations from the Neuromyelitis Optica Study Group (NEMOS) stipulate that both AQP4-IgG and MOG-IgG antibodies must be tested by CBA. The assays must employ cells expressing conformationally intact, full-length recombinant human AQP4 or MOG protein, respectively, as the antigenic substrate and include a control substrate, preferably mock-transfected cells. Other methods such as immunohistochemistry, ELISA, fluorescence-based immunoprecipitation, western blot, and RIA are less sensitive and specific and should no longer be employed for routine clinical testing.
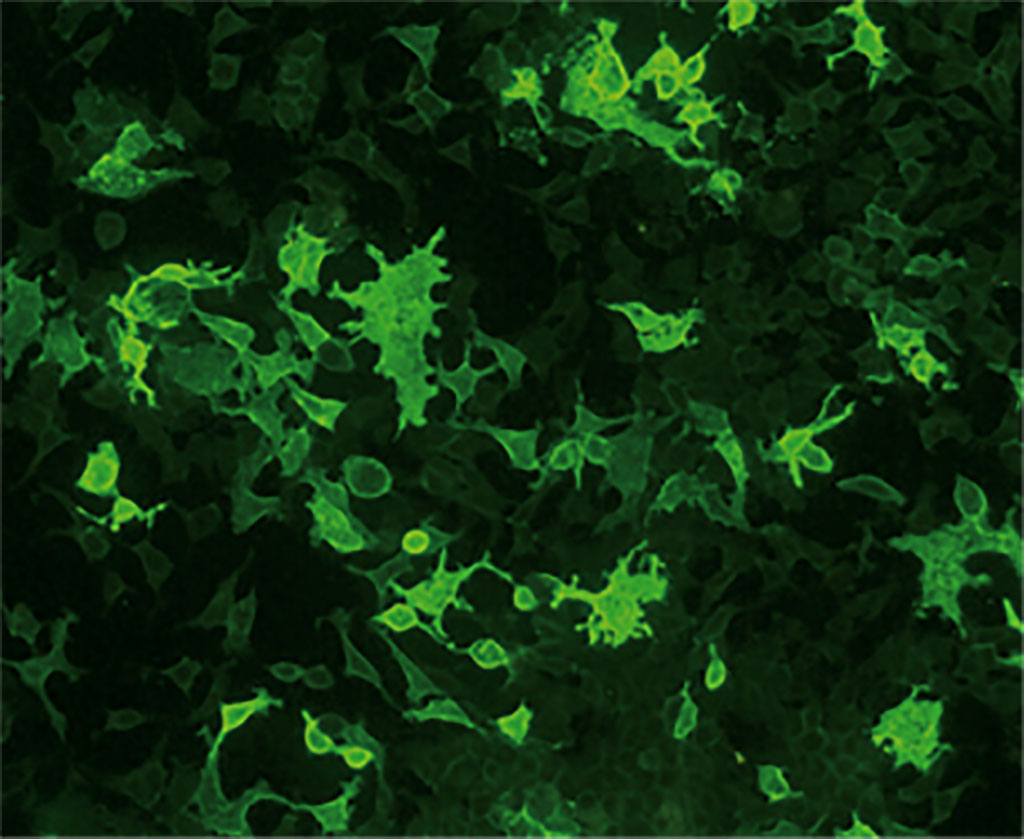
CBAs for AQP4 and MOG antibodies from EUROIMMUN (Lübeck, Germany) meet the recommended requirements. The assays are based on transfected human cell lines expressing the corresponding full-length target antigen and include a control-transfected substrate which is incubated in parallel. EUROIMMUN’s CBA technology enables intact presentation of the fragile conformation-dependent antigens, allowing highly sensitive antibody detection. Moreover, the company’s BIOCHIP Mosaic technology enables easy parallel incubation of different substrates. Miniature sections of the antigen-expressing cells and control-transfected cells are positioned side by side in the fields of a microscope slide and incubated with one patient sample.
Further, AQP4 and MOG antibodies can be analyzed simultaneously using a mosaic containing both AQP4- and MOG-transfected cell substrates together with a control. The EUROIMMUN NMOSD Screen 1 is currently the only commercially available multiparameter CBA for AQP4-IgG and MOG-IgG. All EUROIMMUN assays for anti-AQP4 and anti-MOG antibodies are CE-marked. The analysis procedure is simple and can be performed in any laboratory familiar with indirect immunofluorescence. Automation options are available to facilitate the processing and evaluation. The tests are validated for automated microscopy using the EUROPattern Microscope and the EUROPattern Microscope Live.
Related Links:
EUROIMMUN














