Innovative Blood Test to Revolutionize Prostate Cancer Detection and Reduce Invasive Biopsies
Posted on 03 Oct 2023
One in six men will receive a prostate cancer diagnosis during their lives. Thankfully, if caught early, prostate cancer is highly treatable. However, the existing screening process has its limitations. Currently, the prostate-specific antigen (PSA) test is the standard method for initial screening. It measures PSA levels—a protein produced by the prostate—and higher levels often flag the need for further testing, like biopsies. The challenge is that elevated PSA levels could be due to various factors, like vigorous exercise or prostate infection, not just cancer. This can lead to unnecessary, invasive biopsies and treatments, which may have adverse side effects. To address this issue, a new blood diagnostic test has been developed to offer a risk score based on clinical and biological biomarkers that can help improve decision-making following a high PSA test result.
Nanostics (Edmonton, AB, Canada) has launched ClarityDX Prostate, a rapid, clinically validated diagnostic test that takes into account multiple factors, including PSA levels. It employs the ClarityDX machine learning platform to offer a more accurate risk score for aggressive prostate cancer. The test is designed to help patients and their healthcare providers decide if a biopsy is needed after getting a high PSA test result. It uses a unique machine-learning algorithm and examines data from two biological and three clinical biomarkers. Since it relies on lab data from an existing PSA blood test, it can be easily incorporated into the current patient care pathway at a low additional cost.
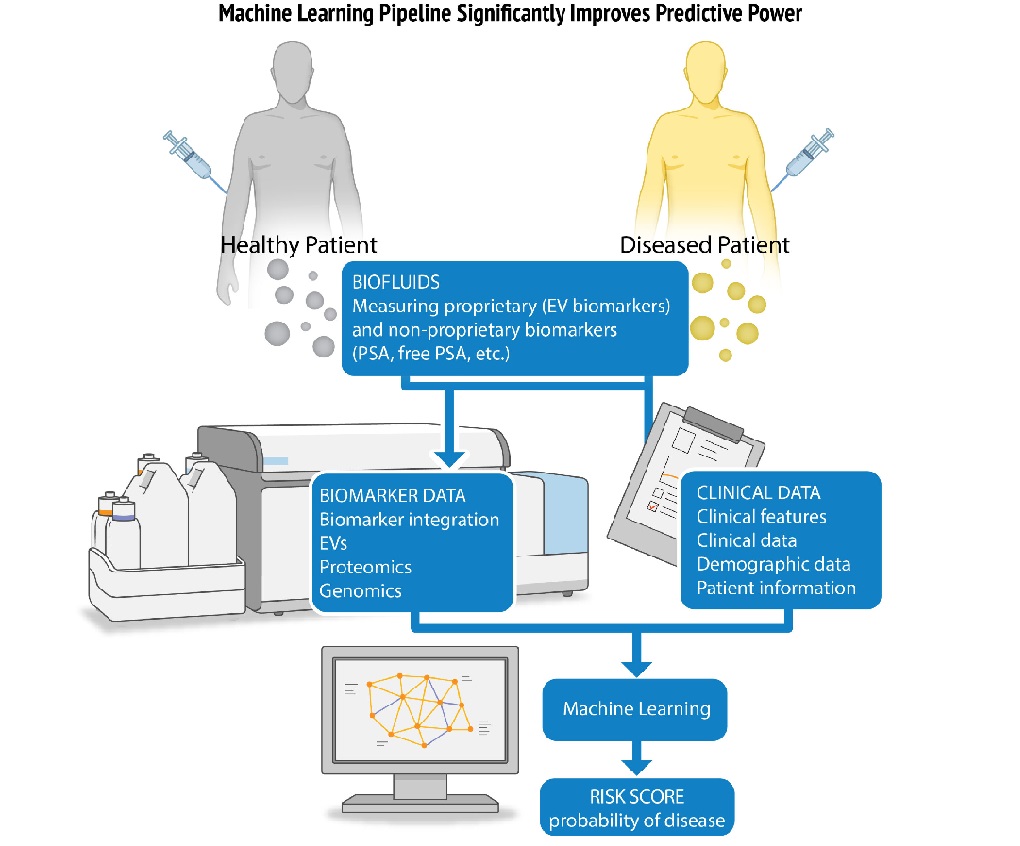
ClarityDX Prostate is particularly useful at the initial diagnosis stage to help decide whether a biopsy is necessary. Studies indicate that adding ClarityDX Prostate to the standard patient care path could reduce unnecessary biopsies by as much as 35%, leading to considerable cost savings. This groundbreaking blood test is a significant step forward in prostate cancer screening. It offers crucial support to men between the ages of 40 and 75 and their doctors in making more informed decisions—three times more accurately—about the need for a biopsy after a high PSA test result.
"This test will reduce the number of unnecessary prostate biopsies, which are invasive, uncomfortable, and carry some risk," said Dr. John D. Lewis, CEO of Nanostics.
Related Links:
Nanostics














