Digital Test Directly Measures HIV Viral Load in Single Drop of Blood
Posted on 22 Jun 2023
One milliliter of blood consists of approximately 15 individual drops. For a person living with human immunodeficiency virus (HIV), each of these drops could house anywhere from less than 20 to over 500,000 viral copies. This measure, known as the viral load, is essential for clinicians to evaluate the response to anti-viral drugs and monitor potential disease progression. However, viral load testing, which must be repeated several times during treatment, can be time-consuming. Researchers have now designed a digital assay that can effectively measure HIV presence in a single blood drop, offering a more time and cost-efficient solution. This assay lays the groundwork for diagnostic tools for a wide range of infectious diseases.
Traditional HIV viral load tests require genetic material extraction from the sample, which is then amplified and compared to reference samples. The gold standard RT-PCR test can approximate the actual viral load, but it doesn't measure it directly. Researchers at Penn State (University Park, PA, USA) devised a more direct method with their Self-digitization Through Automated Membrane-based Partitioning (STAMP) test, which is less expensive, quicker, and requires less blood than the RT-PCR. The method allows the researchers to extract viral RNA (the virus's genetic material) from a small blood sample and mix it with a unique protein called Cas13, part of the CRISPR system. CRISPR-Cas13 is renowned for enabling RNA sequence targeting and manipulation. The researchers use it not just for its editing abilities, but also for its diagnostic potential. The researchers are employing CRISPR-Cas13 to detect and signal the presence of HIV.
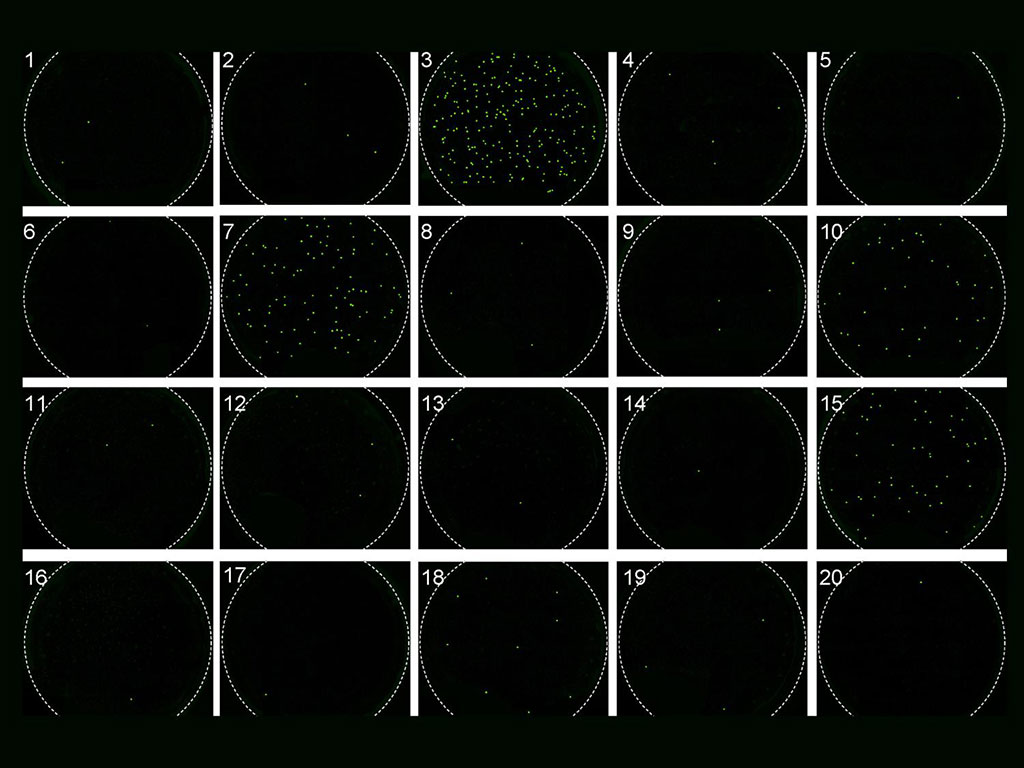
After the RNA is combined with Cas13, the researchers place a nanopore polycarbonate membrane - a readily available, cost-effective material - on the mixture. The nanopores are so minute that they can separate the mixture into single droplets, each containing only one RNA molecule linked to the Cas13 protein. If HIV is present in the RNA molecule, the Cas13 protein - which HIV RNA activates - will cut reporting molecules, creating a detectable signal. The number of droplets indicating this signal helps researchers determine the HIV quantity in the blood. More signal-bearing droplets mean a higher viral load. The team tested this method using synthetic HIV RNA to optimize sensitivity and accuracy before testing lab plasma and eventually patient plasma samples. The STAMP method was validated by measuring HIV viral loads in 20 patient plasma samples, showing comparable accuracy to the traditional RT-PCR method, which generally requires more blood.
The researchers are in the process of securing more samples for further testing. They also found that their approach could accurately assess HIV viral loads at or above approximately 2,000 virus copies per milliliter of blood. Viral loads below 10,000 copies per milliliter are considered low; those at 20 copies or fewer are undetectable; and high viral loads are around 100,000 copies, potentially extending beyond a million. According to the team, the detection range of 2,000 to 10,000 can be crucial for clinicians monitoring patients for viral rebound. Individuals undergoing antiretroviral therapy (ART) can reach undetectable virus levels, making them non-infectious sexually, but an increase in virus levels could suggest ART resistance development or other issues.
“While further improvements are needed to enhance its detection limit and automate the setup, the STAMP-based digital CRISPR method shows great potential for advancing HIV viral load monitoring,” said corresponding author Weihua Guan, stating that the researchers will continue to enhance the platform’s efficiency and accuracy to quantify multiple viruses, with the ultimate goal of bringing the device to market.
Related Links:
Penn State














