Novel Blood Serum Assay Enables Quicker Diagnosis of Neurodegenerative Diseases
Posted on 31 May 2023
Synucleinopathies, a class of neurodegenerative diseases, are caused due to the unusual accumulation of α-synuclein, a protein commonly found in the brain and neurons. Misfolded α-synuclein causes the formation of 'seeds', which draw more of the same protein to create larger clusters. Even though these α-synuclein seeds have been discovered in diverse tissues and blood of patients with synucleinopathies, their utility as a biomarker remains uncertain. Now, scientists have developed a novel assay capable of effectively identifying α-synuclein seeds in a patient's serum.
The assay, named immunoprecipitation-based real-time quaking-induced conversion (IP/RT-QuIC), was developed by scientists at the Juntendo University School of Medicine (Tokyo, Japan). In this process, α-synuclein seeds from the patient's serum are first isolated through immunoprecipitation (a method that uses an antibody that binds exclusively to the target protein for protein separation) and then quickly amplified by real-time quaking-induced conversion (which involves vigorous shaking for amplification). This technique is highly precise and can identify serum α-synuclein seed concentrations as minute as 1000pg/ml. This development is significant because most current diagnostic methods need cerebrospinal fluid for synuclein detection.
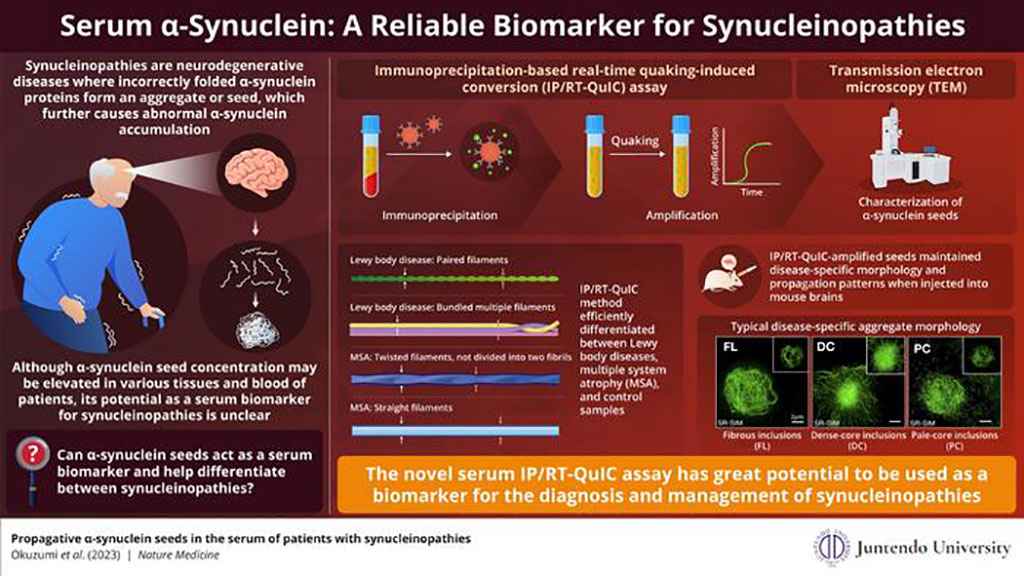
The research team demonstrated that IP/RT-QuIC could successfully identify α-synuclein seeds in patients with neurodegenerative diseases as well as differentiate them from individuals without these diseases. They then analyzed the structural properties of the amplified seeds using transmission electron microscopy (TEM). They found that the structure of synuclein seeds varied based on the type of synucleinopathy. Seeds associated with Parkinson's disease and Lewy bodies displayed paired filaments, while those related to MSA had two unique structures – twisted and straight filaments. This confirmed that IP/RT-QuIC coupled with TEM could distinguish synucleinopathies based on the structure of disease-specific seeds.
Moreover, the researchers discovered that when the amplified seeds were transduced into the HEK293T cell line stably expressing GFP-fused human α-synuclein with p.A53T mutation in a laboratory setting (in vitro) and injected into mouse brains (in vivo), the seeds retained their capacity to form aggregates and disease-specific seed structure. These aggregates exhibited different morphologies based on the type of disease. This suggests that IP/RT-QuIC can diagnose specific synucleinopathies by discerning the structural differences between the α-synuclein seeds and their aggregates. This method has the potential to provide a quick and effective diagnosis for patients.
“At present, a neurologist's consultation is necessary to diagnose synucleinopathies. However, using IP/RTQuIC, a general internist can make the diagnosis,” said Professor Nobutaka Hattori from Juntendo University Faculty of Medicine/RIKEN Center for Brain Science. “Therefore, more patients with synucleinopathies may be diagnosed with precision and could receive appropriate treatment at an earlier stage.”
“Our new IP/RT-QuIC assay may have many future applications as a biomarker for precise diagnosis and monitoring of treatment of neurodegenerative diseases in clinical trials. This simple diagnostic method will enable establishment of personalized therapy options for synucleinopathies,” concluded the researchers.
Related Links:
Juntendo University School of Medicine














