AI-Powered Test Predicts Risk of Developing Esophageal Cancer in Patients with Barrett’s Esophagus
Posted on 16 May 2023
Barrett's esophagus (BE) is a serious consequence of gastroesophageal reflux disease (GERD) and presents a risk for the onset of esophageal cancer, one of the fastest-increasing cancers in terms of incidence in the U.S., exhibiting a less than 20% five-year survival rate. Effective interventions such as ablation therapy are typically applied to patients with higher grades of BE to prevent its progression to esophageal cancer. However, a substantial number of BE patients are classified with lower grades of BE or as non-dysplastic. Despite population-based predictions indicating a low cancer development probability for these lower-grade patients, they constitute the majority of patients who ultimately progress to esophageal cancer. This discrepancy indicates a significant clinical need for personalized, biologically based risk-stratification information to ensure a better alignment between progression risk and the application of effective interventions.
Castle Biosciences’ (Pittsburgh, PA, USA) TissueCypher Barrett’s Esophagus test is the world’s first precision medicine test that predicts the future development of esophageal cancer in patients with BE. The TissueCypher test provides medical professionals with vital information regarding an individual patient's risk of progression to esophageal cancer based on advanced analysis of biopsy samples, thus enabling better-informed, risk-aligned patient management. Its clinical effectiveness has been validated through nine peer-reviewed publications involving BE progressor patients across leading clinical centers worldwide.
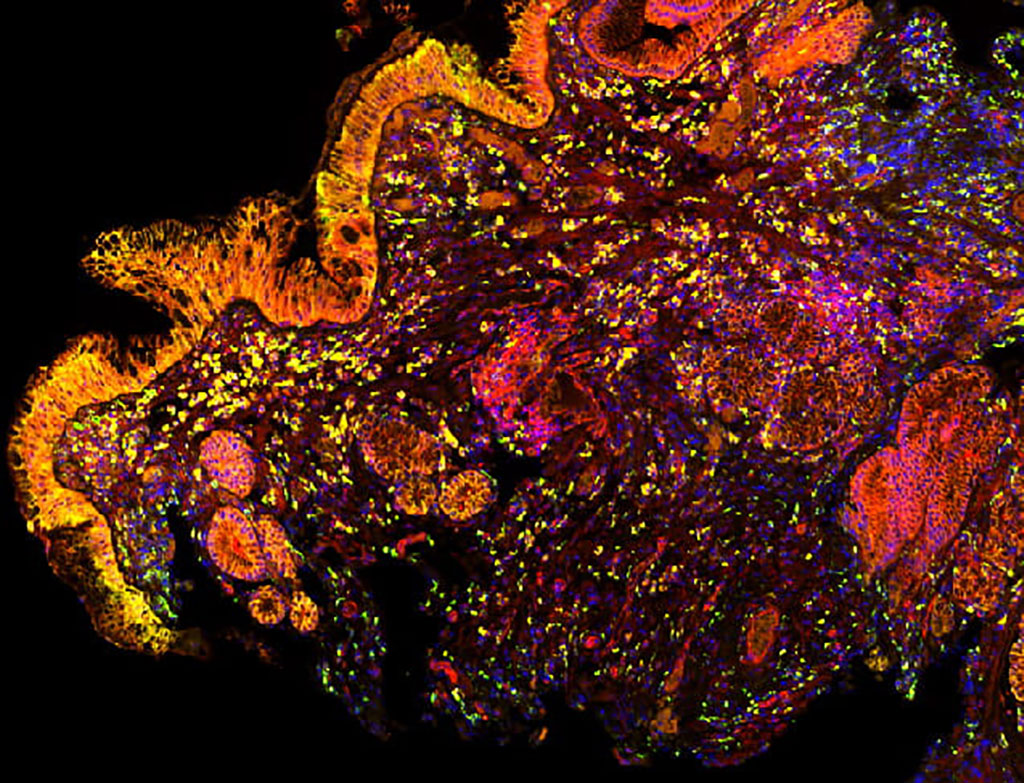
The TissueCypher test is designed for use in patients with endoscopic biopsy-confirmed BE that is graded as non-dysplastic, indefinite for dysplasia, or low-grade dysplasia. Precise and accurate prediction of progression from BE to high-grade dysplasia or esophageal adenocarcinoma is crucial, given the rapidly growing incidence of esophageal cancer. Since esophageal adenocarcinoma is highly fatal once diagnosed, early detection and advanced warning provided by the TissueCypher test in BE patients can offer crucial clinical decision support for physicians treating these patients. Castle Biosciences was selected as the winner of the “Best Use of Artificial Intelligence in Healthcare” award in the seventh annual MedTech Breakthrough Awards program for its innovative TissueCypher Barrett’s Esophagus test.
“Most cancer diagnoses and associated risk-stratification estimates are currently made by pathologists viewing tissue on glass slides via light microscopy. This approach is limited in its ability to evaluate multiple biomarkers and cell types within the tumor system and predict future development of cancer,” said James Johnson, managing director, MedTech Breakthrough. “TissueCypher utilizes artificial intelligence to predict the risk of developing esophageal cancer – one of the world’s most deadly cancers. Congratulations to the Castle team on winning the ‘Best Use of Artificial Intelligence in Healthcare’ award in 2023.”
Related Links:
Castle Biosciences














