New Diagnostic Test 1,000 Times More Sensitive than Conventional Tests
Posted on 13 Feb 2023
A key challenge in the field of infectious disease diagnostics is to quickly find out if a patient has a bacterial infection and needs antibiotics or has a viral infection for which antibiotics are not effective. Despite significant advances made in infectious disease diagnostics, there is still a need for simple, rapid and sensitive tests that can provide quantitative information and can be employed in sophisticated laboratories or in the field. In their effort to overcome the limitations of point-of-care diagnostic tests, researchers have now developed ultrabright fluorescent nanolabels called plasmonic-fluors, which can be quickly integrated into the lateral flow assay (LFA), a common testing platform. Plasmon-enhanced LFAs (p-LFAs) improve inexpensive, readily available rapid tests to the levels of sensitivity required by physicians for confidence in test results without the need for lab-based confirmation.
The p-LFAs designed by the research team at Washington University in St. Louis (WUSTL, St. Louis, MO, USA) are 1,000 times more sensitive than conventional LFAs, which show results via a visual color and fluorescence signal on the strip. When analyzed using a fluorescence scanner, p-LFAs are also significantly faster than gold-standard lab tests and return results within just 20 minutes instead of several hours, with equal or improved sensitivity. The p-LFAs can detect and quantify concentrations of proteins, enabling them to detect bacterial and viral infections as well as markers of inflammation that indicate other diseases. The improved testing capability fulfills the present need for quick and reliable test results and also eliminates the risk of false negatives. After proving that p-LFAs can outperform standard lab tests in sensitivity, speed, convenience and cost for one disease, the researchers now plan to develop new applications for the technology, including identifying bacterial versus viral infections and making their diagnostic tool accessible to physicians across the world.
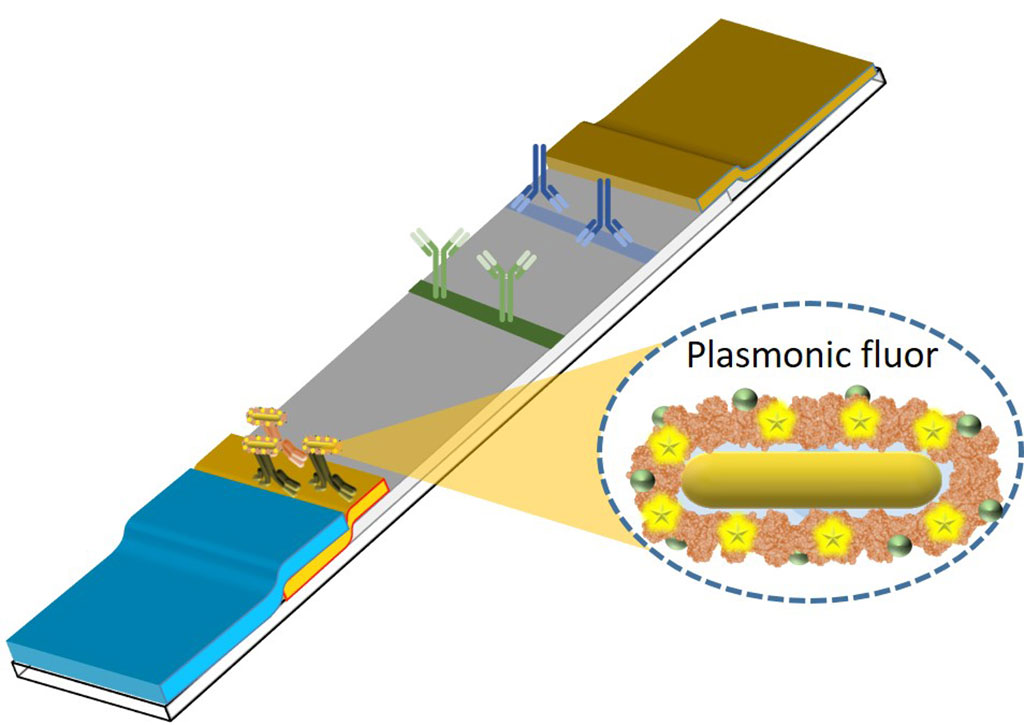
“Our p-LFAs can pick up even very small concentrations of antibodies and antigens, typical markers of infection, and give clinicians clear, quick results without the need for specialized equipment,” explained Srikanth Singamaneni, a professor of mechanical engineering and materials science at the McKelvey School of Engineering at WUSTL. “For quantitative testing beyond the initial screening, the same LFA strip can be scanned with a fluorescence reader, enabling rapid and ultrasensitive colorimetric and fluorometric detection of disease markers with only one test.”
“We expect to have p-LFAs commercially available in the next one to two years,” Singamaneni said. “Right now, we’re working on improving our portable scanner technology, which adds a more sensitive, fluorescent reading capability to the test strips in addition to the color change that can be seen with the naked eye. We think we can get that cost down to a point where it’s accessible to rural clinics in the U.S. and abroad, which was one of our original goals.”
“We’re also excited about the potential to detect many more diseases than COVID, possibly using a skin patch that can take a painless sample,” Singamaneni added. “This technology has the potential to detect any number of diseases, ranging from STIs to respiratory infections and more, as well as cytokines indicative of inflammation seen in conditions such as rheumatoid arthritis and sepsis.”
Related Links:
WUSTL













