Late-Onset Ataxia Linked to Repeat Expansion in FGF14 Gene
Posted on 21 Dec 2022
There are many causes of cerebellar ataxia including, among others, gluten ataxia, autoimmunity to Purkinje cells or other neural cells in the cerebellum, CNS vasculitis, multiple sclerosis, infection, bleeding, infarction, tumors, direct injury, toxins, genetic disorders and neurodegenerative diseases (such as progressive supranuclear palsy and multiple system atrophy).
Late-onset cerebellar ataxias (LOCAs) are a heterogeneous group of neurodegenerative disorders manifesting as a progressive cerebellar syndrome that develops after 30 years of age. The prevalence of LOCA is approximately 1 to 3 per 100,000 population, and molecular testing yields negative results in almost 75% of patients with LOCA. This is explained in part by the limitations of standard next-generation sequencing analysis for the identification of certain sequence variations, such as tandem repeat expansions.
A large team of scientists at McGill University (Montreal, QC, Canada), and the University of Miami's Miller School of Medicine (Miami, FL, USA) and other colleagues sequenced the genomes of six persons with autosomal dominant LOCA who were members of three French Canadian families and identified a candidate pathogenic repeat expansion. They then tested for association between the repeat expansion and disease in two independent case–control series: one French Canadian (66 patients and 209 controls) and the other German (228 patients and 199 controls). They also genotyped the repeat in 20 Australian and 31 Indian index patients. They assayed gene and protein expression in two postmortem cerebellum specimens and two induced pluripotent stem-cell (iPSC)–derived motor-neuron cell lines.
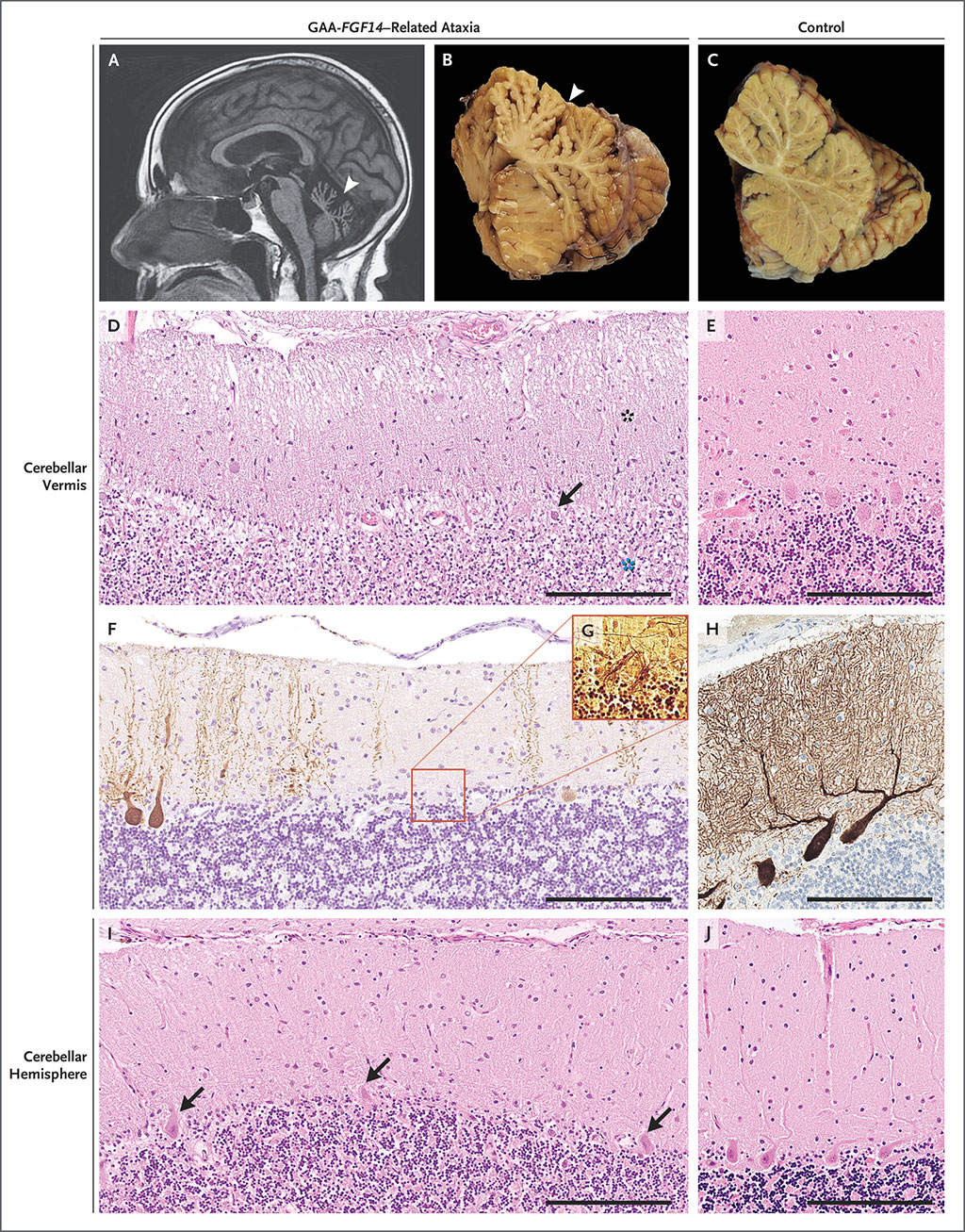
The FGF14 repeat locus was amplified by long-range polymerase chain reaction (PCR). The number of repeat units was estimated by means of agarose-gel electrophoresis. The motif of the repeat locus in patients and controls that had large amplification products on long-range PCR was analyzed by targeted long-read nanopore sequencing. They tested for association between LOCA and FGF14 GAA expansions of 250 or more repeats ([GAA]≥250), as measured by agarose-gel electrophoresis of PCR-amplification products. Postmortem cerebellar tissue was obtained from two patients of Spanish origin who carried an FGF14 GAA repeat expansion that had been uncovered after screening of postmortem brain specimens from 15 patients of European descent with unsolved LOCA and seven controls of European descent without ataxia.
The investigators reported that in the six French Canadian patients, they identified a GAA repeat expansion deep in the first intron of FGF14, which encodes fibroblast growth factor 14. Co-segregation of the repeat expansion with disease in the families supported a pathogenic threshold of at least 250 GAA repeats. They detected the repeat expansion in late-onset cerebellar ataxia patients from other populations, albeit in a smaller proportion of cases. They saw the FGF14 repeat expansion in 18% of the German cases and 15% of the Australian cases, but just 10% of late-onset cerebellar ataxia cases from India. In total, they identified 128 patients with LOCA who carried an FGF14 (GAA)≥250 expansion. Postmortem cerebellum specimens and iPSC-derived motor neurons from patients showed reduced expression of FGF14 RNA and protein.
The authors concluded that they had identified a dominant GAA repeat expansion in the first intron of FGF14 in persons with unsolved LOCA. The findings also show how late-onset disorders can be associated with individual alleles of strong genetic effect. The study underscores the importance of identifying noncoding repeat expansions, because they probably account for some of the missing heritability in unsolved neurodegenerative disorders. The study was published on December 14, 2022 in the New England Journal of Medicine.
Related Links:
McGill University
University of Miami's Miller School of Medicine














