Clinical Multiplex Benchtop Diagnostic System Runs Tests Directly From Whole Blood
Posted on 24 May 2022
A fully automated, walk away, clinical multiplex benchtop diagnostic system is capable of running tests directly from whole blood.
The T2Dx Instrument from T2 Biosystems (Lexington, MA, USA) is powered by the company’s T2 Magnetic Resonance (T2MR) technology which measures how water molecules react in the presence of magnetic fields. Over 200 studies published in peer-reviewed journals have featured T2MR in a breadth of applications, including direct detection and measurement in targets such as whole blood, plasma, serum, saliva, sputum, and urine. T2MR is the first and only detection technology that can quickly and accurately identify molecular targets within patient samples without the need for purification or extraction of target molecules from the sample. T2MR eliminates these time- and labor-intensive steps. The T2MR diagnostic signal is not compromised or disrupted by the sample background, even for highly complex sample backgrounds present in blood from patients suspected of having sepsis.
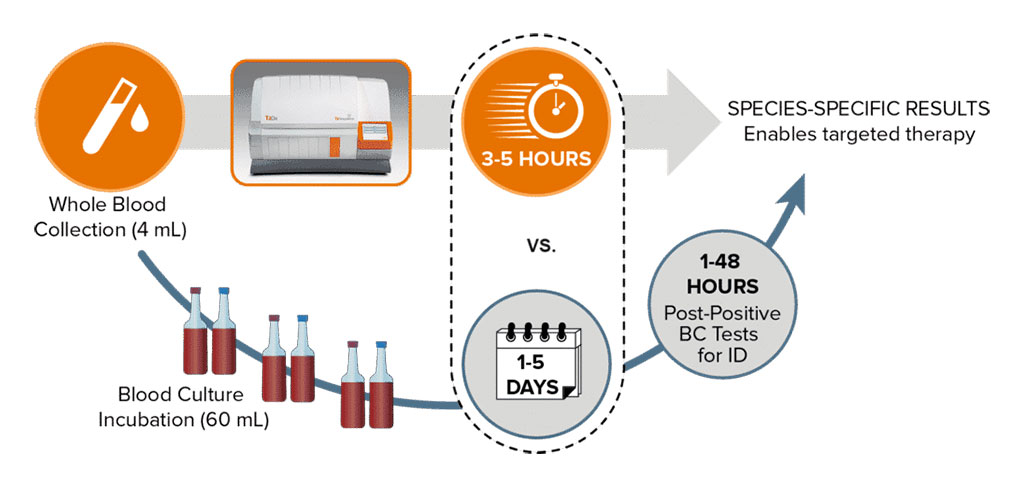
The number of cells required for blood culture positivity is typically in the range of 10,000 to 1,000,000 CFUs/mL. This is more than a thousand-fold increase in the number of cells required for T2MR detection direct from whole blood. The T2MR-powered T2Dx Instrument can detect organisms as low as 1 CFU/mL, enabling it to be the only FDA-cleared technology that can detect low levels of pathogens in whole blood. When operating the fully automated system with minimal hands-on time, a sample tube and a reagent tray snap onto a cartridge, which is then inserted into the instrument. No blood culture is required to receive T2 identification results for targeted pathogens and the T2Dx Instrument delivers species-specific results in 3 to 5 hours with no up-front sample preparation.
It allows seven individual, random access drawers to be loaded at any time and eliminates the need for batching. Its user-friendly touchscreen display provides step-by-step text and illustrative prompts that guide the operator to load a sample, all in 10 minutes or less. The T2Dx Instrument serves nearly 200 hospitals worldwide and runs the FDA-cleared T2Bacteria and T2Candida Panels as well as the T2SARS-CoV-2 Panel, which was awarded Emergency Use Authorization (EUA) by the FDA.
“T2 Biosystems is the only company with solutions that can detect sepsis-causing pathogens directly from whole blood in hours instead of days,” said John Sperzel, Chairman and Chief Executive Officer of T2 Biosystems. “With the mortality of patients in septic shock rising by 8% every hour they are not on targeted treatment, the need for hospitals to have more effective rapid diagnostics that enable earlier targeted therapy integrated into their infection management protocol is essential.”
Related Links:
T2 Biosystems














