A Device for Rapid and Accurate Diagnosis of Hepatitis C Infections
By LabMedica International staff writers
Posted on 07 Dec 2021
A device has been developed that enables the rapid and accurate diagnosis of hepatitis C infections under both laboratory and field conditions.Posted on 07 Dec 2021
Hepatitis C virus (HCV) is a major cause of liver-related disease with more than 70 million people chronically infected globally. Recent advances in direct acting antiviral treatments have improved cure rates to better than 95%. However, currently an estimated 80% of all infected individuals are unaware of their status due to the asymptomatic nature of infection. Many of these patients will not be diagnosed until irreversible clinical damage, such as liver cirrhosis and hepatocellular carcinoma, occur - syndromes that contribute to the more than 400,000 HCV related deaths reported every year.
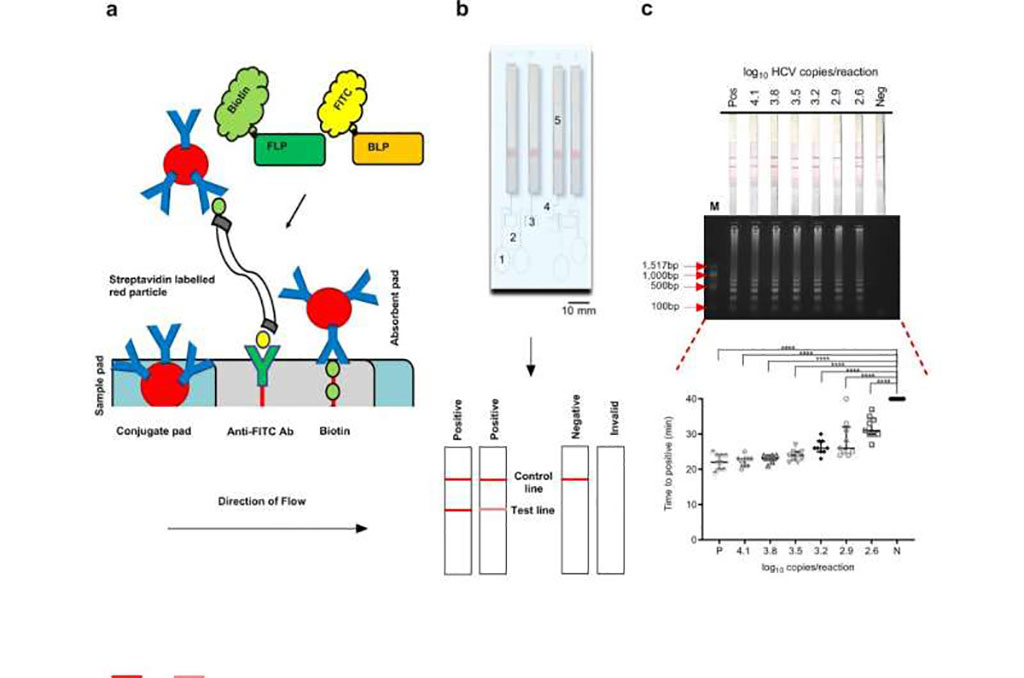
Image: Lateral flow detection of hepatitis C virus (HCV) using a novel LAMP assay (Photo courtesy of Nature Communications at www.nature.com)
Currently, testing for HCV is hindered by high cost, long turnaround times, and high level of expertise needed in centralized diagnostic laboratories. To rectify this situation, investigators at the University of Glasgow (United Kingdom) developed a user-friendly, low-cost assay based on reverse transcriptase loop mediated isothermal amplification (RT-LAMP).
LAMP assays provide high sensitivity and specificity through the use of four to six primers, which target six to eight regions within a sequence of interest. The amplification reaction takes place at a constant temperature between 60–65 degrees Celsius, offering a cheaper alternative to the traditional PCR assays, with minimal equipment requirements.
The new device for HCV detection, which was adapted from a similar system that had been developed to deliver rapid malaria diagnosis, incorporated sheets of origami-like folded wax paper to prepare samples for LAMP amplification. The nature of the folded paper enabled the sample to be processed and delivered to three small chambers in a cartridge, which the LAMP machine heated and used to test the samples for the presence of hepatitis C RNA. The results were delivered in the form of an easy-to-read lateral flow strip with two bands for a positive result and one band for a negative.
The prototype device, with potential for point-of-care use, described in the current study comprised a LAMP amplification chamber and lateral flow nucleic acid detection strips, giving a visually-read, user-friendly result in less than 40 minutes.
To verify the performance of the prototype, the investigators analyzed 100 blood plasma samples from patients with chronic HCV infection and another 100 samples from a control group of HCV-negative patients. Results were compared to those obtained for the same samples using an Abbott (Abbott Park, IL, USA) RealTime hepatitis C assay. Results of the LAMP analyses were found to be in 98% agreement with those from the Abbott test.
Senior author Dr. Jonathan Cooper, professor of biomedical engineering at the University of Glasgow, said, “The World Health Organization has published guidelines for the kinds of rapid, accurate diagnostic tests which could help tackle infectious diseases around the world, including hepatitis C. Our malaria diagnosis system was a response to that call to action. While that tested patients' blood for the presence of the DNA of Plasmodium falciparum, the mosquito-borne parasitic species which causes malaria, we were confident that it could be adapted for other purposes.”
The rapid diagnostic device for diagnosis of hepatitis C infections was described in the November 30, 2021, online edition of the journal Nature Communications.
Related Links:
University of Glasgow
Abbott













