Next-Generation Sequencing Impacts All Aspects of Myelodysplastic Syndrome Care
By LabMedica International staff writers
Posted on 25 Nov 2021
Myelodysplastic Syndrome (MDS) is a type of blood cancer that affects the bone marrow. It causes low levels of one or more types of blood cells in the blood. MDS is more common in people aged over 70, but it can happen at any age.Posted on 25 Nov 2021
Signs and symptoms of MDS may include dizziness, fatigue, weakness, shortness of breath, bruising and bleeding, frequent infections, and headaches. Management of myelodysplastic syndromes is most often intended to slow the disease, ease symptoms and prevent complications. Common measures include blood transfusions and medications to boost blood cell production.
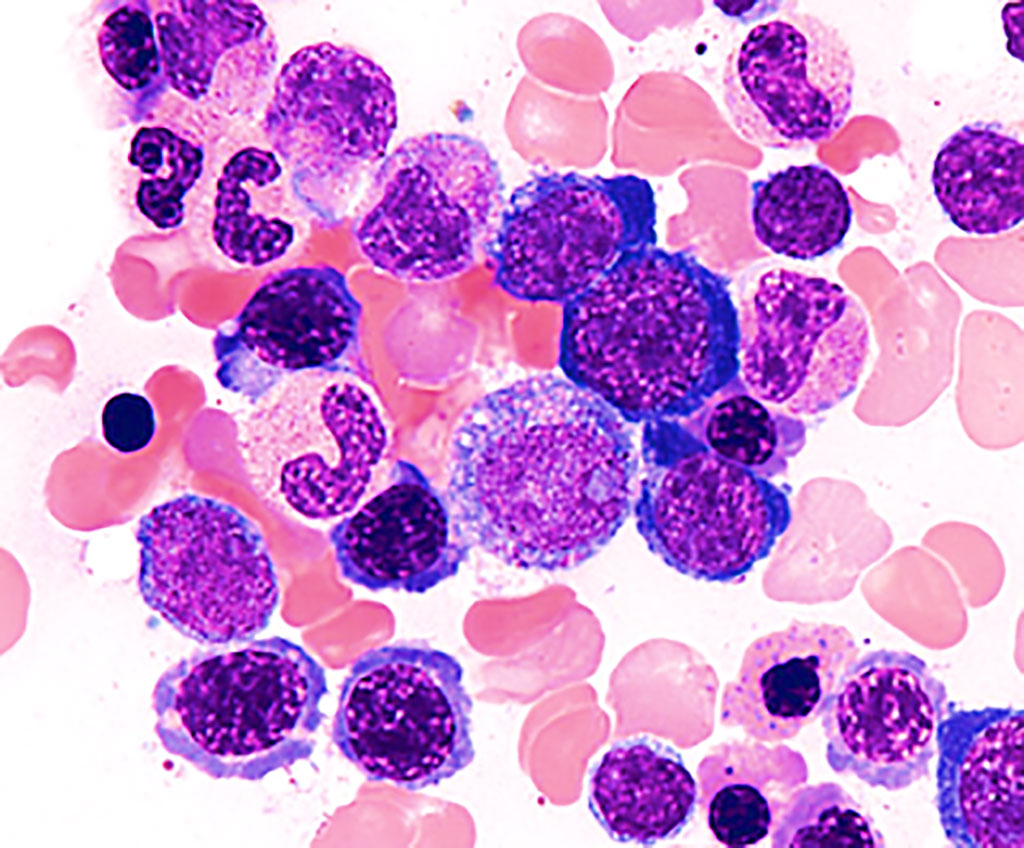
Image: Bone marrow smear from a person with myelodysplastic syndrome showing aberrant morphology and maturation (dysmyelopoiesis), resulting in ineffective blood cell production (Photo courtesy of Melbourne Blood Specialists)
Hematologists at the Moffitt Cancer Center (Tampa, FL, USA) and their colleagues incorporated next-generation sequencing into standard of practice that will be required for patients with myelodysplastic syndrome and will impact all facets of care. They highlighted TP53 mutations to show the importance of personalization, particularly for subgroups that respond poorly to standard-of-care therapy. They identified TP53 mutation status and variant allele frequency as predictors of survival among patients with myelodysplastic syndrome and secondary acute myeloid leukemia. The very adverse-risk group, which represents the majority of patients, consists of those with a high variant allele frequency of 40%, complex karyotype or more than one mutation, or a mutation in the setting of TP53.
The scientists noted that clinicians can wait for results of sequencing panels for the vast majority of patients with myelodysplastic syndrome, adding that turnaround time continues to improve. Meanwhile, other factors that can provide a high pretest probability of whether a patient may have a TP53 mutation include therapy-related history, multiple abnormalities, refractory anemia excess blasts with increased ringed sideroblasts and p53 immunohistochemistry. Other mutations that potentially could be targeted include IDH1 and IDH2 mutations, which are rare in myelodysplastic syndrome, and splicing mutations.
To improve outcomes for this molecular subset of patients, scientists are investigating eprenetapopt, a first-in-class p53 reactivator. Results of a phase 2 study showed the agent in combination with azacitidine induced responses in more than 70% of patients, with complete remission rates of 40% to 50%. The phase 3 study, however, did not meet its primary endpoint of improved complete remission, as other trials of novel combinations that include the agent are ongoing and the investigators remains hopeful.
David A. Sallman, MD, a Hematologist and lead author of the study, said, “We really think going forward, particularly in the setting of novel therapy, that achievement of p53 clearance, as low as possible, potentially at that point then bridging to transplant, may be the ultimate approach.” The study was presented at the 39th Annual Chemotherapy Foundation Symposium held November 3-5, 2021 in New York, NY, USA.
Related Links:
Moffitt Cancer Center













