New Biomarker Improves Definition of Medulloblastoma Risk
By LabMedica International staff writers
Posted on 08 Nov 2021
An immunohistochemistry marker has been identified as being able to improve risk stratification of medulloblastoma, the most common form of pediatric brain tumor, using a technique that is within the capabilities of most clinical laboratories.Posted on 08 Nov 2021
The World Health Organization (WHO) classification system recognize eight molecular subgroups among Group 3/4 medulloblastoma, representing about 60% of tumors. However, very few clinical centers worldwide possess the technical capabilities to determine DNA-methylation profiles or other molecular parameters of high-risk for Group 3/4 tumors.
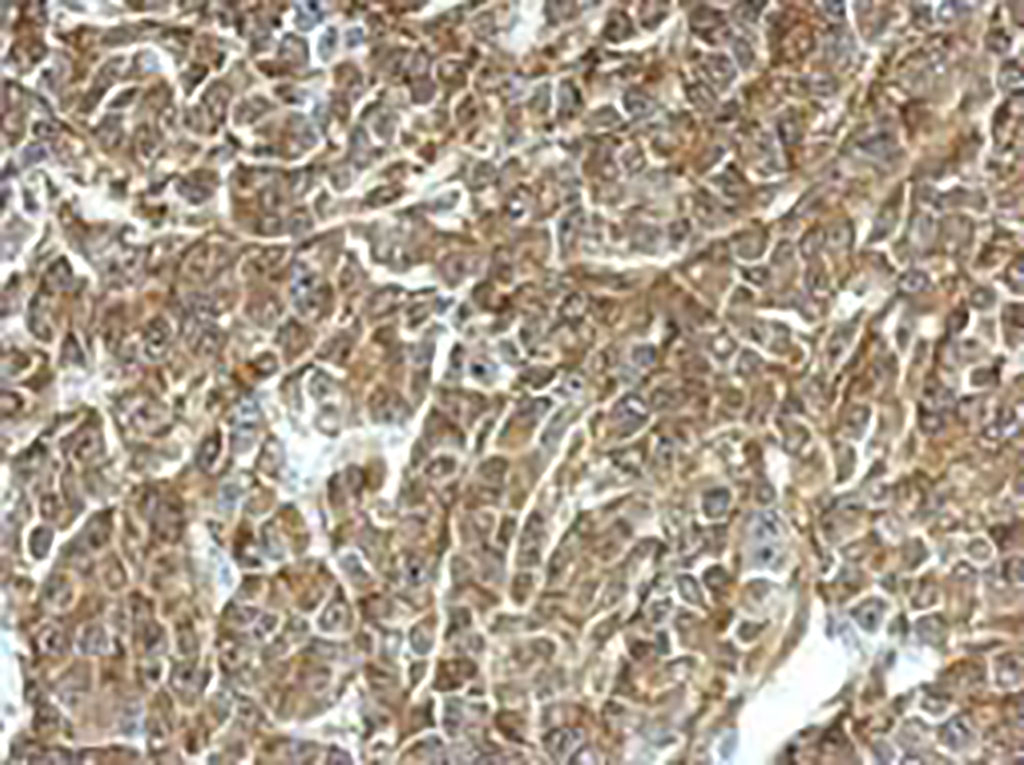
Image: Immunohistochemical analysis using a labeled antibody for TPD52 recognition (Photo courtesy of GeneTex)
To bring this classification system within reach of most clinical laboratories, investigators at The University of British Columbia (Vancouver, Canada) turned to the widely used antibody-based technique, immunohistochemistry (IHC).
Initially, the investigators used a bioinformatics approach to analyze published medulloblastoma transcriptomes and proteomes. This approach allowed them to identify the protein TPD52 (Tumor protein D52) as a potential biomarker.
The investigators then screened samples from 387 medulloblastoma patients who were being treated with conventional therapies for presence of TPD52. Results revealed that TPD52 IHC positivity represented a significant independent predictor of early relapse and death for Group 3/4 medulloblastoma. Cross-validated survival models incorporating TPD52 IHC with clinical features outperformed existing state-of-the-art risk stratification schemes, and reclassified nearly 50% of patients into more appropriate risk categories. Finally, TPD52 immunopositivity was a predictive indicator of poor response to chemotherapy.
“With this new test, more doctors may one day be able to identify children with the most aggressive forms of medulloblastoma and better tailor treatment,” said senior author Dr. Poul Sorensen, professor of pathology and laboratory medicine at The University of British Columbia.
The study was published in the October 26, 2021, online edition of the journal Clinical Cancer Research.
Related Links:
The University of British Columbia










 Analyzer.jpg)



