Biohit’s Innovative GastroPanel Quick Test Receives CE Mark
Posted on 15 Oct 2021
Biohit Healthcare’s (Helsinki, Finland) GastroPanel Quick Test, the latest innovation in its unique GastroPanel product family, is now CE marked.
The GastroPanel Quick Test is intended for diagnosing Helicobacter pylori infection and atrophic gastritis in patients with dyspeptic symptoms. The test measures pepsinogen I, pepsinogen II, gastrin-17 and antibodies against Helicobacter pylori from human plasma sample in one test. The test also identifies people at a risk of developing malignant cellular changes in stomach mucosa or who may require additional stomach examination or treatment. The test results are available in 20 minutes, enabling fast diagnosis and screening for further examinations.
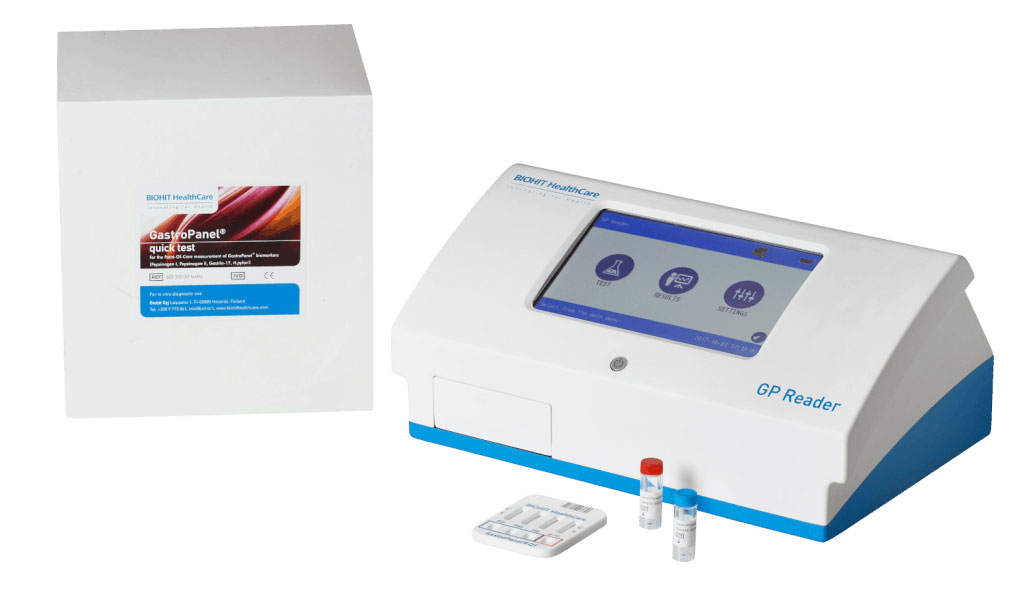
The GastroPanel Quick Test system comprises of an immunological test and dedicated GP Reader device which interprets the result. The clinical performance of the GastroPanel quick test system is in line with the on market available and previously validated ELISA-based GastroPanel test. In a study, samples collected from 500 patients with upper abdominal symptoms were tested with the GastroPanel Quick Test. The results were compared with gastric endoscopies and biopsies performed according to international guidelines. The concordance between the methods was demonstrated to be excellent.
“We are excited to launch the GastroPanel Quick Test system with such excellent performance characteristics and fast turnaround time,” said Osmo Suovaniemi, the acting CEO of Biohit. “We believe that the test system will be well received by end users because the usability of the test system enables first-line diagnosis and screening of dyspeptic patients easier than ever before. We are eager to continue developing the testing system further. Next, we will extend the CE mark of the GastroPanel Quick Test to include finger-prick blood samples by the end of 2021.”
Related Links:
Biohit Healthcare













