Saliva-Based Liquid Biopsy Detects HPV-Caused Head and Neck Cancers
By LabMedica International staff writers
Posted on 27 Sep 2021
A saliva-based liquid biopsy assay was developed that might eventually play an essential role in the detection and management of head and neck cancers caused by high-risk human papillomaviruses.Posted on 27 Sep 2021
High-risk human papillomavirus (HR-HPV) infection is a major risk factor of head and neck cancers (HNCs). These cancers constitute 3–5% of all malignancies worldwide and there are approximately 600,000 newly diagnosed cases annually. Patients are usually diagnosed at an advanced stage, since in the early stages, these cancers are difficult to locate with imaging studies or physical examination. Furthermore, the oropharynx is difficult to access, and detection is complicated if these smaller lesions are hidden in the crevices of the tonsils. Importantly, these cancers can metastasize at an early stage, even when the primary cancer is still undetectable in size. Despite the rising prevalence of HPV-driven HNC (HPV-HNC) and closely associated oropharyngeal cancers (OPC), biomarkers for detection, prognostication, and disease monitoring are lacking.
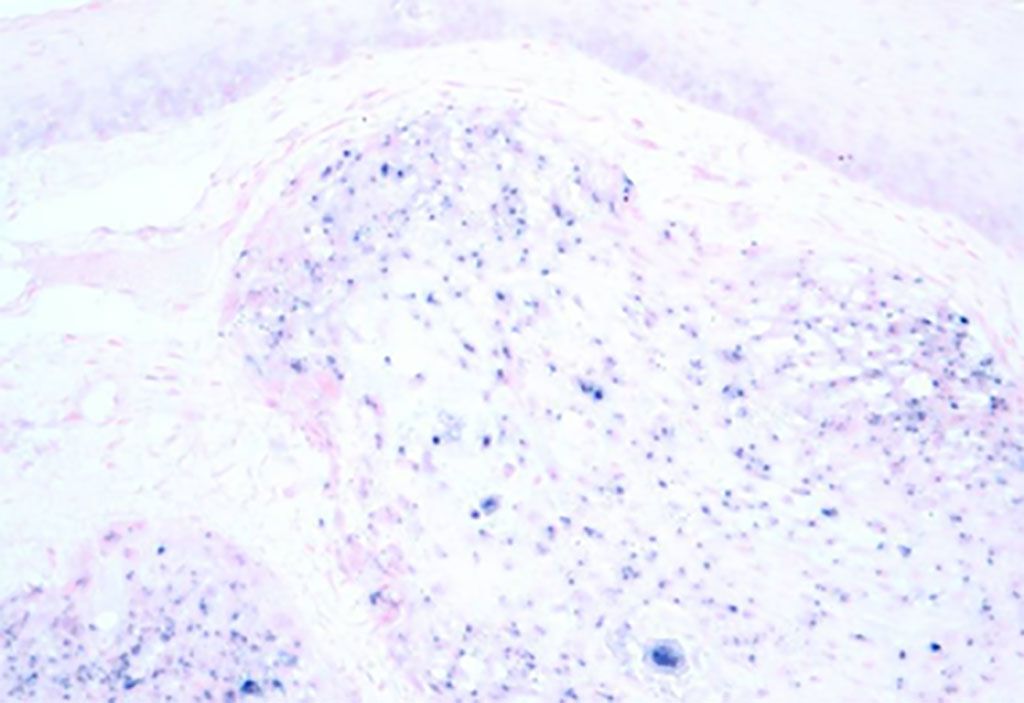
Image: Micrograph image of human papilloma virus associated oropharyngeal cancer (HPV+ OPC). The tissue was stained to show the presence of the virus by in situ hybridization (Photo courtesy of Wikimedia Commons)
Investigators at Queensland University of Technology (Brisbane, Australia) performed a study to evaluate the potential use of salivary HR-HPV DNA as a biomarker of HPV-HNC. For this study, they employed quantitative real-time PCR and the Agena Bioscience (San Diego, CA, USA) MassARRAY system to analyze saliva samples obtained from 491 patients at the time of first diagnosis of HNC and 10 patients with recurring HNC. An additional group of OPC patients was monitored for a period of up to five years.
Results revealed that of the primary-HNC cohort, 43.2% were positive for salivary HR-HPV DNA, with most having OPC. Salivary HR-HPV DNA was detected in 81.4% of tumor p16–positive OPC patients at diagnosis. Salivary HR-HPV–positive patients had a clear survival advantage over their salivary HR-HPV–negative counterparts. The median event-free survivals were 205 months for HR-HPV–positive patients, compared to 82 months for HR-HPV–negative patients.
“Despite the upsurge in HPV-driven HNC, there are no early detection methods or screening strategies for this cancer type, unlike cervical cancer, which is caused by the same virus. Biomarkers enabling early detection, monitoring, and disease prognostication are warranted to combat the rising incidence of HPV-driven OPC,” said senior author Dr. Chamindie Punyadeera, associate professor of biomedical sciences at Queensland University of Technology. “When the noninvasive nature and convenience of the collection are considered, salivary HR-HPV testing is an ideal mode of screening asymptomatic individuals and the long-term monitoring of HPV-driven HNC patients. Our findings indicate that in the near future, salivary HR-HPV testing will become part of routine clinical management for HPV-driven OPC patients.”
The saliva-based biopsy was described in the July 26, 2021, online edition of The Journal of Molecular Diagnostics.
Related Links:
Queensland University of Technology
Agena Bioscience














