Liquid Biopsy Assay Evaluated for Early Detection of Gastric Cancer
By LabMedica International staff writers
Posted on 08 Sep 2021
Gastric cancer (GC) is the fourth most-commonly diagnosed cancer, and the third leading cause of cancer-associated mortality worldwide. Despite improvements in treatment modalities, the prognosis for advanced GC following curative resection remains poor.Posted on 08 Sep 2021
Patients diagnosed with early-stage GC have a favorable prognosis, underscoring the paradigm that identification at earlier stages remains an attractive strategy for reducing GC-associated patient mortality. The use of liquid biopsy-based, noninvasive cancer biomarkers has become increasingly desirable, and several promising molecular biomarkers have been identified in blood, urine, and gastric juice.
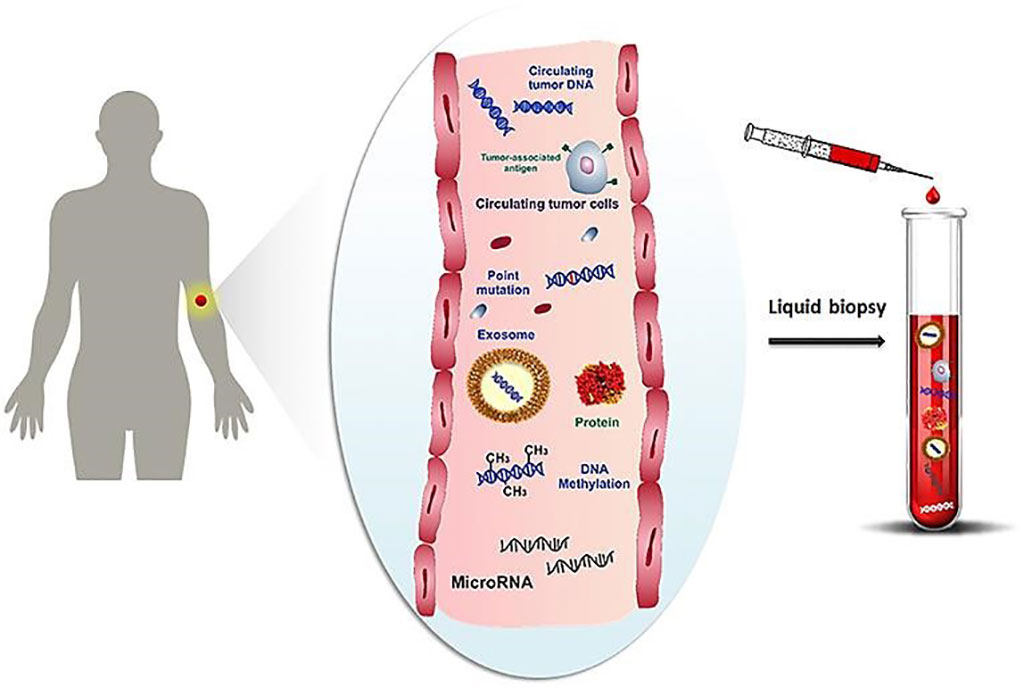
Image: Schematic representation of cancer-related biomolecules such as cells, proteins, nucleic acids, miRNA and microvesicles circulating into the bloodstream, and collection of these biomarkers by liquid biopsy (Photo courtesy of University of Florence)
Gastroenterologists at the Baylor University Medical Center (Dallas, TX) and their international colleagues analyzed more than 1,900 tissue and serum specimens from patients with GC, adjacent normal tissues, and healthy participants across four phases. Study phases included a biomarker discovery phase, a tissue validation phase, a retrospective serum validation phase, and a prospective serum performance evaluation phase.
The biomarker discovery cohort (436 GC tissues and 41 adjacent normal mucosae) was analyzed to identify miRNA candidates. In the tissue validation phase, quantitative reverse-transcription–polymerase chain reaction (qRT-PCR) assays were performed to interrogate the expression levels of candidate miRNAs in 50 pairs of matched, fresh-frozen, primary tumor and adjacent normal tissues from patients with GC. In the prospective serum validation phase, serum specimens were collected from 176 patients with GC and 173 healthy participants, matched by age and sex, who were prospectively recruited from March 2017 to August 2018. The 10 miRNAs were validated in two additional independent datasets that included 40 GC and 40 non-cancerous tissue specimens with miRNA profiling data acquired using the miRNA microarray (Agilent Technologies, Santa Clara, CA, USA).
The scientists reported that the data sets for the genome-wide expression profiling analysis stage included 598 total patient samples (284 [55.4%] from men; mean ±SE patient age, 65.7 ± 0.5 years). The resulting 10-miRNA signature was validated in two retrospective GC serum cohorts (586 patients; 348 [59.4%] men, mean ± SE age, 66.0 ± 0.7 years), which led to the establishment of a 5-miRNA signature (AUC, 0.90) that also exhibited high levels of diagnostic performance in patients with stage I disease (AUC, 0.89) A risk-scoring model was derived and the assay was optimized to a minimal number of miRNAs. The performance of the resulting 3-miRNA signature was then validated in a prospective cohort of 349 patients with GC.
The final 3-miRNA signature (miR-18a, miR-181b, and miR-335) exhibited high diagnostic accuracy in all stages of patients (AUC, 0.86), including in patients with stage I disease (AUC, 0.85). This miRNA signature was superior to currently used blood markers and outperformed the endoscopic screening in a cost-effectiveness analysis (incremental cost-effectiveness ratio [USD 2,304.80 per quality-adjusted life-year]).
The authors concluded that their study established a robust, noninvasive, circulating miRNA signature for GC detection, and validated its diagnostic potential in multiple independent patient cohorts, both retrospective and prospective, highlighting its potential application for the early detection of patients with GC. The study was published on August 24, 2021 in the journal JAMA Network Open.
Related Links:
Baylor University Medical Center
Agilent Technologies