CSF biomarkers Compared for Down Syndrome and Inherited Alzheimer’s
By LabMedica International staff writers
Posted on 19 Aug 2021
Although most people with Down’s syndrome will experience brain changes as they age, not everyone will develop Alzheimer’s disease or another type of dementia. Whilst having Down’s syndrome does put a person at increased risk, estimated at 1 in 3 people in their 50s, and closer to 2 in 3 aged over 60, it is not inevitable for all.Posted on 19 Aug 2021
Due to trisomy of chromosome 21 and the resultant extra copy of the amyloid precursor protein gene, nearly all adults with Down syndrome develop Alzheimer's disease pathology by the age of 40 years and are at high risk for dementia given their increased life expectancy compared with adults with Down syndrome in the past.
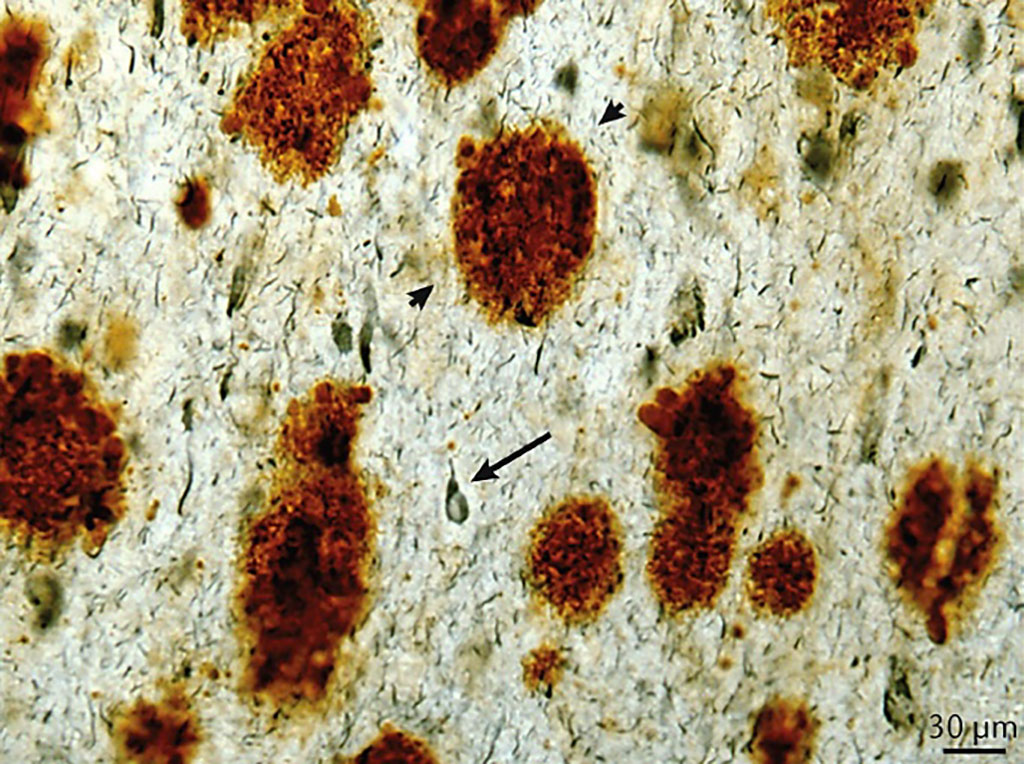
Image: Representative example of amyloid-β plaques (Aβ1-42; brown staining; arrowheads) and neurofibrillary tangles (PHF1 antibody; blue staining; arrow) in the frontal cortex of a 46-year-old person with Down syndrome and end-stage Alzheimer disease. (Photo courtesy of University of California, Irvine).
A large team of Neuroscientists led by those at Washington University School of Medicine (St. Louis, MO, USA) and their colleagues carried out a cross-sectional study that included 341 individuals (178 [52%] women, 163 [48%] men, aged 30–61 years). Participants were 41 adults with Down syndrome, similarly aged carriers of autosomal dominant Alzheimer's disease mutations (n=192), and non-carrier siblings (n=108). Participants with baseline cerebrospinal fluid (CSF), available clinical diagnosis, and apolipoprotein E genotype as of January 31, 2019, were included in the analysis.
CSF samples obtained from adults with Down syndrome, similarly aged carriers of autosomal dominant Alzheimer's disease mutations, and non-carrier siblings (aged 30–61 years) were analyzed for markers of amyloid β (Aβ1–40, Aβ1–42); tau phosphorylated at threonine 181-related processes; neuronal, axonal, or synaptic injury (total tau, visinin-like protein 1, neurofilament light chain [NfL], synaptosomal-associated protein 25); and astrogliosis and neuroinflammation (chitinase-3-like protein 1 [YKL-40]) via immunoassay.
The team reported that individuals with Down syndrome had patterns of Alzheimer's disease-related CSF biomarkers remarkably similar to carriers of autosomal dominant Alzheimer's disease mutations, including reductions in Aβ 1-42 to Aβ 1-40 ratio and increases in markers of phosphorylated tau-related processes; neuronal, axonal, and synaptic injury; and astrogliosis and neuroinflammation, with greater degrees of abnormality in individuals with dementia. Differences included overall higher concentrations of Aβ and YKL-40 in Down syndrome and potential elevations in CSF tau and NfL in the asymptomatic stage (i.e., no dementia symptoms). The study was published in the August edition of the journal Lancet Neurology.
Related Links:
Washington University School of Medicine














