Genomic Copy Number Predicts Esophageal Cancer Years Before Transformation
By LabMedica International staff writers
Posted on 23 Sep 2020
Barrett’s esophagus is a condition in which the lining of the esophagus changes, becoming more like the lining of the small intestine rather than the esophagus. This occurs in the area where the esophagus is joined to the stomach. Posted on 23 Sep 2020
Most patients with Barrett’s esophagus will not develop cancer. In some patients, however, a precancerous change in the tissue, called dysplasia, will develop. That precancerous change is more likely to develop into esophageal cancer.
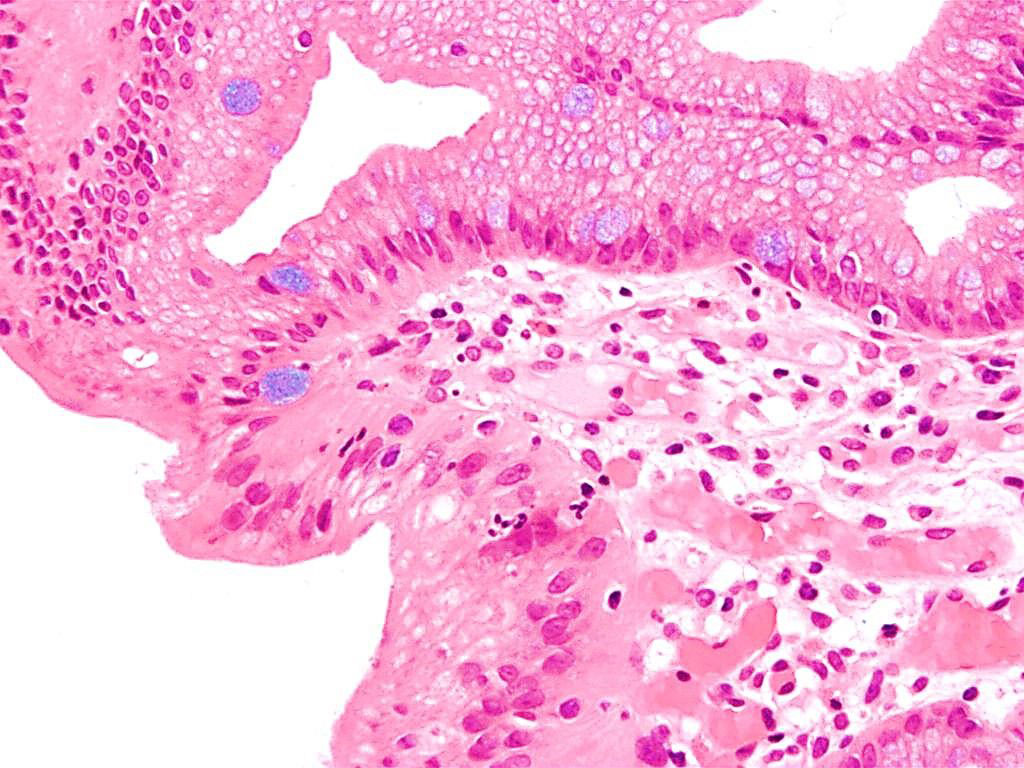
Image: Photomicrograph of histopathology of Barrett`s esophagus showing the characteristic goblet cells (Alcian blue stain) (Photo courtesy of Nephron).
A team of scientists from the University of Cambridge (Cambridge, UK) assessed whether these genomic signals can be used for early detection and pre-emptive cancer treatment using the neoplastic precursor lesion Barrett’s esophagus as an exemplar. They conducted shallow whole-genome sequencing on a retrospective case-control cohort of 88 patients. More than 770 endoscopy samples had been collected from the patients during clinical surveillance of Barrett's esophagus. The team used shallow whole-genome sequencing as it not only gave a genome-wide view of copy number changes but also had been optimized for use on formalin-fixed paraffin-embedded samples.
Overall, they found that samples from patients whose disease progressed to cancer exhibited generalized disorder across their genomes. Based on the copy number data they generated and a measure of overall complexity, the team developed an elastic-net-regularized logistic regression model of progression and classification of disease. They validated the model in an independent cohort of 76 patients and orthogonally validated it using single nucleotide polymorphism (SNP) array samples from 248 patients. Slightly more than half the samples (55%) from patients who did not progress were classified as low risk using the investigator’s model. At the same time, 77% of the samples from patients who did progress were classified as high risk.
When analyzed in conjunction with current Barrett's esophagus management guidelines, the scientists estimated that their approach would have led 54% of patients who progressed to receive earlier treatment. Meanwhile, they also estimated that of the patients who did not progress, 51% would have had less frequent endoscopies if their model had been applied.
Rebecca C. Fitzgerald, MD, FMedSci, a Professor of Cancer Prevention and senior author of the study, said, “This demonstrates that genomic risk stratification has a realistic potential to enable earlier intervention for high-risk conditions, and at the same time reduce the intensity of monitoring and even reduce overtreatment in cases of stable disease.”
The authors concluded their methods are low-cost and applicable to standard clinical biopsy samples. Compared with current management guidelines based on histopathology and clinical presentation, genomic classification enables earlier treatment for high-risk patients as well as reduction of unnecessary treatment and monitoring for patients who are unlikely to develop cancer. The study was published on September 7, 2020 in the journal Nature Medicine.
Related Links:
University of Cambridge














