A Novel Liquid Biopsy Device Enables Early Cancer Detection and Diagnosis
By LabMedica International staff writers
Posted on 22 Jun 2020
A novel liquid biopsy device for early cancer detection and diagnosis was used to isolate and analyze extracellular vesicles from breast cancer tumors.Posted on 22 Jun 2020
Evidence has accumulated, which indicates that extracellular vesicles (EVs) have important functions in tumor progression and metastasis, including matrix remodeling via transporting matrix metalloproteases (MMPs).
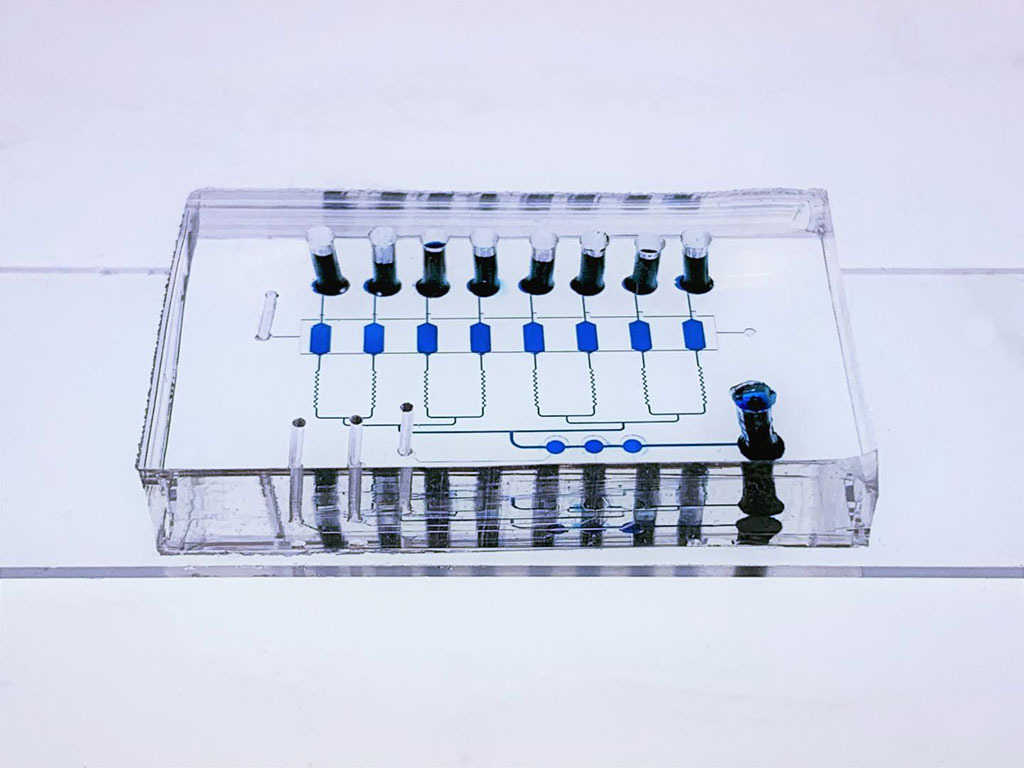
Image: The multi-layer EV-CLUE chip device. The microreactors and connecting channels are visualized by filling with blue food dye. The bottom glass slide is patterned with nanoparticle structures and coated with antibody to capture extracellular vesicles (Photo courtesy of Dr. Yong Zeng)
Proteins of the matrix metalloproteinase (MMP) family are involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction, and tissue remodeling, as well as in disease processes, such as arthritis and metastasis.
In the meantime, the clinical relevance of EVs has remained largely undetermined, partially owing to challenges in EV analysis. EVs, which contain RNA, proteins, lipids, and metabolites that are reflective of the cell type of origin, are increasingly being recognized as important vehicles of communication between cells and as promising diagnostic and prognostic biomarkers in cancer. Despite this huge clinical potential, the wide variety of methods for separating EVs from biofluids, which provide material of highly variable purity, and the lack of knowledge regarding methodological reproducibility have impeded the entry of EVs into the clinical arena.
To open up the clinical potential for analysis of EVs, investigators at the University of Kansas (Lawrence, USA) developed a generalized, high-resolution colloidal inkjet printing method that allowed robust and scalable manufacturing of three-dimensional nanopatterned devices. These nanopatterned polydimethylsiloxane/glass microfluidic chips (EV-CLUE chips) were used to analyze EVs in plasma. The chips captured EVs expressing different surface markers of interest and measured the expression and activity of the EV-bound enzyme MMP14.
The EV-CLUE chip is a multi-layer device constructed by stacking two slabs made of polydimethylsiloxane (PDMS) on a glass slide. The top PDMS slab was microfabricated with a network of pressure/vacuum valves and pump that controlled the circuit of eight parallel microreactors engraved on the middle thin PDMS layer. The bottom glass slide was patterned with nanoparticle structures and coated with antibody to capture extracellular vesicles.
Analysis of clinical plasma specimens showed that EV-CLUE technology could be used for cancer detection including accurate classification of age-matched controls and patients with ductal carcinoma in situ, invasive ductal carcinoma, or locally metastatic breast cancer in a training cohort (n = 30, 96.7% accuracy) and an independent validation cohort (n = 70, 92.9% accuracy).
The investigators expect that their EV-CLUE technology will provide a useful liquid biopsy tool to improve cancer diagnostics and real-time surveillance of tumor evolution in patients, which would be another step on the road to truly personalized cancer therapy.
The EV-CLUE device was described in the June 10, 2020, online edition of the journal Science Translational Medicine.
Related Links:
University of Kansas














