Phosphorylated Tau Protein Blood Test Distinguishes Alzheimer’s Disease from Other Neurological Disorders
By LabMedica International staff writers
Posted on 04 May 2020
A recently developed blood test has confirmed the hypothesis that elevated levels of circulating phosphorylated tau 181 (pTau181) protein correlate with Alzheimer's disease (AD) and distinguish it from other neurological disorders that may be misdiagnosed as AD.Posted on 04 May 2020
Since neurological disorders such as frontotemporal dementia are as common as AD among adults under 65 years of age, simple, widely available screening tests are needed to identify which individuals, with symptoms of cognitive or behavioral decline should be further evaluated for initiation of treatment. A blood-based test for AD would be a less invasive and less expensive screening tool than the currently approved cerebrospinal fluid or amyloid-beta positron emission tomography (PET) diagnostic tests.
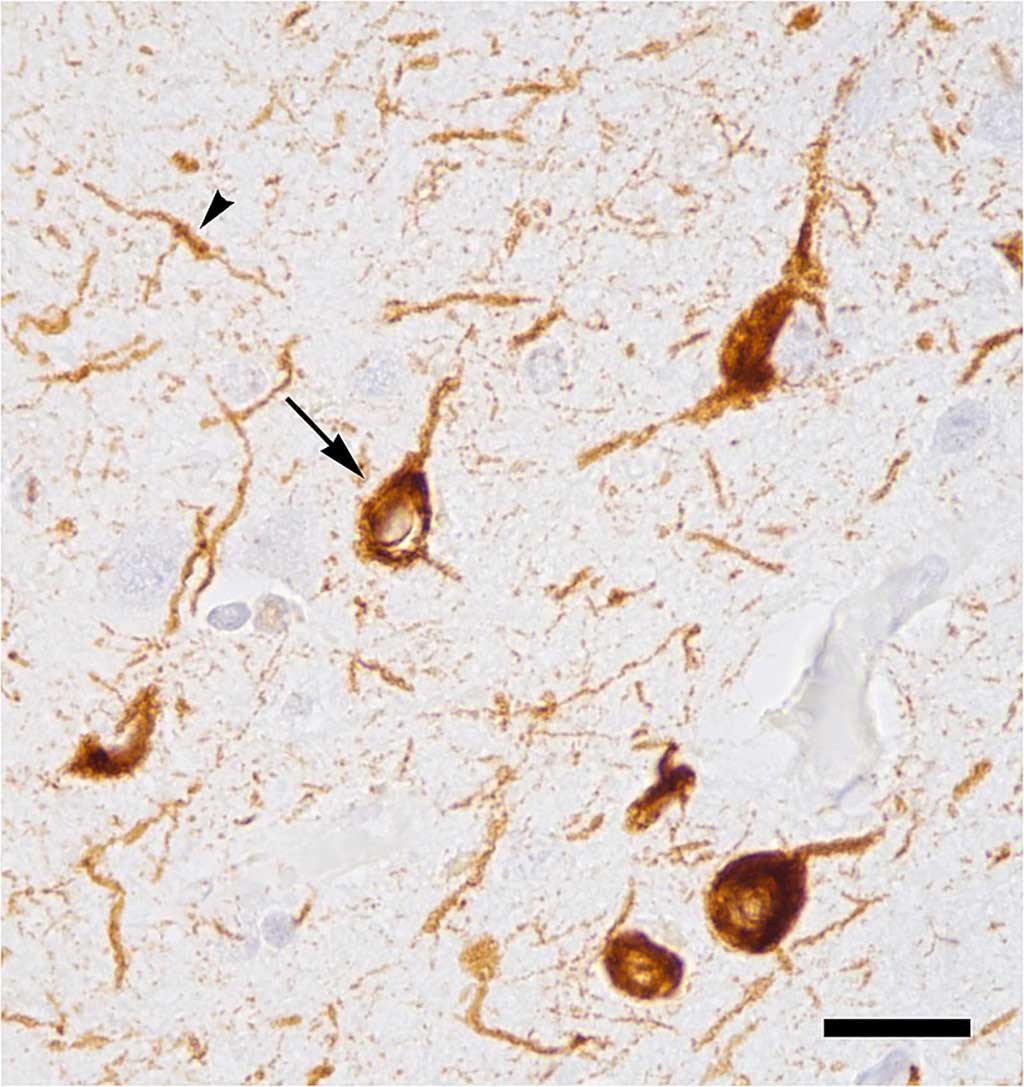
Image: Abnormal accumulation of tau protein in neuronal cell bodies (arrow) and neuronal extensions (arrowhead) in the neocortex of a patient who had died with Alzheimer`s disease (Photo courtesy of Wikimedia Commons).
In an effort to develop a blood-based test for AD, investigators at the University of Gothenburg (Sweden) examined whether plasma tau protein phosphorylated at residue 181 (pTau181) could differentiate between clinically diagnosed or autopsy-confirmed AD and other neurological disorders.
For this purpose, the investigators adapted an assay based on measurement of pTau181 in ordinary blood samples using the extremely sensitive Single Molecule Array (Simoa) technique. The Simoa method can detect considerably lower levels of protein biomarkers than other analytical methods.
The current study included four clinic-based prospective cohorts. The discovery cohort comprised 37 patients with Alzheimer's disease and age-matched controls. Two validation cohorts (226 in TRIAD and 763 in BioFINDER-2) included cognitively unimpaired older adults (mean age 63–69 years), participants with mild cognitive impairment (MCI), Alzheimer's disease, and frontotemporal dementia. In addition, TRIAD included healthy young adults (mean age 23 years) and BioFINDER-2 included patients with other neurodegenerative disorders. The primary care cohort comprised 1131 control participants from the community without a diagnosis of a neurological condition and patients referred from primary care physicians for specialist care.
Results obtained with the Simoa assay revealed that in all cohorts, plasma pTau181 showed gradual increases along the Alzheimer's disease continuum, from the lowest concentrations in amyloid beta-negative young adults and cognitively unimpaired older adults, through higher concentrations in the amyloid beta-positive cognitively unimpaired older adults and MCI groups, to the highest concentrations in the amyloid beta-positive MCI and Alzheimer's disease groups.
Plasma pTau181 distinguished Alzheimer's disease dementia from amyloid beta-negative young adults and cognitively unimpaired older adults, as well as other neurodegenerative disorders (including frontotemporal dementia, vascular dementia, progressive supranuclear palsy, corticobasal syndrome, Parkinson's disease, or multiple systems atrophy). Furthermore, in the primary care cohort, plasma pTau181 discriminated Alzheimer's disease patients from young adults and cognitively unimpaired older adults but not from MCI.
"We believe that, in the future, one very important use of our blood test will be for screening in primary care. We demonstrated this in one of the studies forming part of our article, in which we looked at patients in primary care with concerns about their failing memory," said senior author Dr. Kaj Blennow, professor of clinical neurochemistry at the University of Gothenburg.
The phosphorylated tau 181 assay was described in the May 1, 2020, issue of the journal The Lancet Neurology.
Related Links:
University of Gothenburg













