RT-LAMP Assay Developed for SARS-CoV-2
By LabMedica International staff writers
Posted on 20 Apr 2020
SARS-CoV-2 is the causative viral pathogen of COVID-19, and diagnosis of COVID-19 can be done through CT scan of suspicious patients and a confirmatory laboratory test is performed using published real-time quantitative polymerase chain reaction (RT-qPCR) methods. Posted on 20 Apr 2020
Although RT-qPCR methods are used as the gold-standard for detection of pathogens due to its high sensitivity and specificity, it still have some caveats. To overcome any restriction of RT-qPCR and still detect pathogens’ nucleic acids, isothermal amplification methods have been developed. Among such methods, Loop-mediated isothermal amplification (LAMP) method has some advantages to be applied for point-of-care test (POCT). Well optimized LAMP assay shows sensitivity comparable to that of PCR, less than 10 copies per reaction.
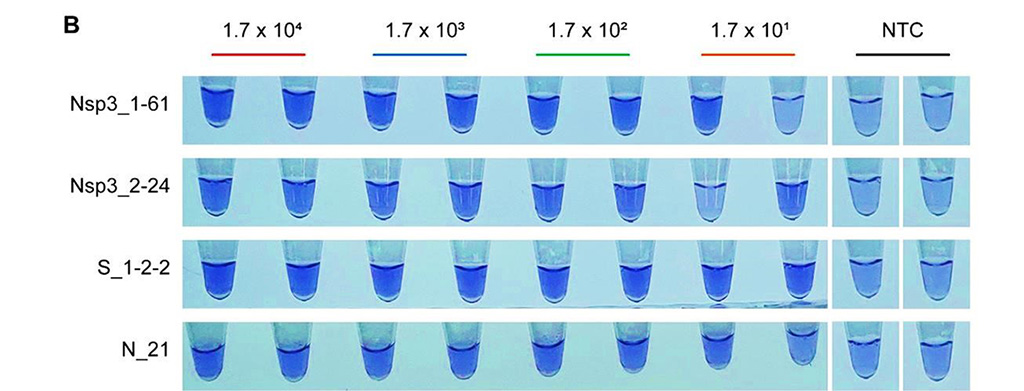
Image: Lauco crystal violet (LCV) colorimetric detection results of limit of detection (LoD) tests for the primer sets used for SARS-CoV-2 detection. 20U/reaction of reverse transcriptase were used (Photo courtesy of Korea Research Institute of Chemical Technology).
Scientists at the Korea Research Institute of Chemical Technology (Daejeon, Republic of Korea) and their colleagues developed and evaluated RT-LAMP assays to detect genomic RNA of SARS-CoV-2. The team used SARS-CoV-2 viral RNA that was prepared as previously described. hCoV-229E and hCoV-OC43 viral RNA were isolated from culture media of infected MRC-5 cells and MERS-CoV RNA was isolated from cell pellet lysate of infected Vero cells. To evaluate genomic copy number of viral RNAs, dilutions of standard RNAs and viral RNAs in TE buffer were subjected to one-step RT-qPCR. RT-qPCR reactions were carried out using a LightCycler 96 instrument (Roche Molecular Systems, Inc, Pleasanton, CA, USA).
The team reported that RT-LAMP assays used in their study can detect as low as 100 copies of SARS-CoV-2 RNA. Five out of seven primer sets showed specific amplification for at least one replicate of duplicate with cDNA concentration corresponding to 1.7 x 101 copies of input RNA. Cross-reactivity of RT-LAMP assays to other human Coronaviruses was not observed. The lauco crystal violet (LCV) method was applied to achieve colorimetric detection of LAMP reaction for their RT-LAMP assay so that the tests potentially performed in higher throughput.
The authors concluded that they have developed highly specific RT-LAMP assays for detection of SARS-CoV-2. The results of these RT-LAMP assays can be detected within 30 minutes after amplification reaction began. In addition, they provided optimized reaction conditions to which LCV colorimetric detection method is applied that can be used for point-of-care tests. The study was published on April 7, 2020 in The Journal of Molecular Diagnostics.













