Annual Blood Tests Recommended for Patients with MGUS
By LabMedica International staff writers
Posted on 29 Jul 2019
Individuals with low-risk or intermediate-risk monoclonal gammopathy of undetermined significance (MGUS) can convert to high-risk MGUS and progress to multiple myeloma within five years, a finding that supports the need for annual blood testing and risk assessment for all individuals with MGUS or light-chain MGUS.Posted on 29 Jul 2019
MGUS is a plasma cell disorder in which plasma cells or other types of antibody-producing cells secrete an abnormal antibody (myeloma protein) into the blood. This abnormal protein is usually found in the blood and/or urine during standard laboratory blood or urine tests. MGUS resembles multiple myeloma and similar diseases, but the levels of antibodies are lower, the number of plasma cells (white blood cells that secrete antibodies) in the bone marrow is lower, and it rarely has symptoms or major problems. However, since MGUS can lead to multiple myeloma, which develops at the rate of about 1.5% a year, yearly monitoring has been recommended.
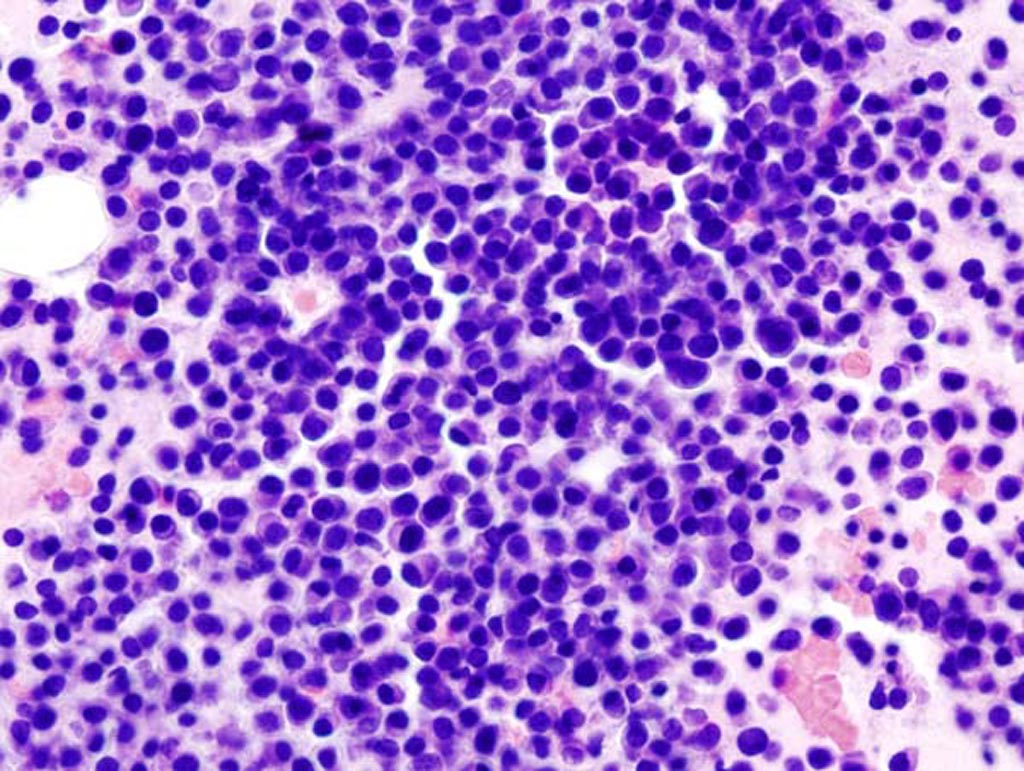
Image: A photomicrograph of bone marrow aspirate showing the histologic correlate of multiple myeloma (Photo courtesy of Wikimedia Commons).
Investigators at Memorial Sloan Kettering Cancer Center (New York, NY, USA) wondered whether it was possible to trace changes in serum immune markers as MGUS progressed to multiple myeloma. To answer this question, the investigators used serial blood samples to examine blood-based immune markers associated with progression from MGUS to multiple myeloma.
The investigators analyzed serum protein and monoclonal immunoglobulin levels, serum free light chains, and serum light chains within each immunoglobulin class in 3266 serum samples from a cohort of 685 individuals with a diagnosis of progressing or stable MGUS. The participants in this analysis were taking part in the National Cancer Institute Prostate, Lung, Colorectal, and Ovarian (NCI-PLCO) cancer screening trial, a prospective study that followed 77,469 people, ages 55 to 74 years old, who were all cancer free at the time of enrollment.
Results of longitudinal analysis of individuals with serial samples prior to progression showed that 23 of 43 (53%) had high-risk MGUS before progression, and 16 of these 23 (70%) experienced conversion from low-risk or intermediate-risk MGUS to multiple myeloma within five years. Similar results were found for light-chain MGUS. Evolving monoclonal proteins, serum-free light chains, and immunosuppression were associated with disease progression.
“This study based on prospectively collected samples helps us to better understand the findings of the prior retrospective studies,” said first author Dr. Ola Landgren, chief of myeloma service at Memorial Sloan Kettering Cancer Center. “Previously reported annual risk of progression from MGUS to multiple myeloma of 0.5% to 1% reflected the average risk among all MGUS cases but were not applicable to individual patients. In the current study, we found that the risk of progression is not constant. Our data indicates that individuals with low-risk or intermediate-risk MGUS can convert to high-risk MGUS and progress to multiple myeloma within a five-year window. This finding has direct clinical relevance and supports annual blood tests for all individuals diagnosed with MGUS or light-chain MGUS, and, importantly, yearly re-assessment of a patient’s clinical-risk status.”
The study was published in the July 18, 2019, online edition of the journal JAMA Oncology.
Related Links:
Memorial Sloan Kettering Cancer Center














