New Test Predicts Potential Success of BCG Immunotherapy for Bladder Cancer
By LabMedica International staff writers
Posted on 03 Sep 2018
A laboratory test that measures the amount of IL2 secretion from CD4+ T cells after overnight in vitro incubation with tuberculin predicts the potential success of BCG (Bacille Calmette-Guérin) immunotherapy treatment for bladder cancer.Posted on 03 Sep 2018
BCG is used in the treatment of superficial forms of bladder cancer. Since the late 1970s, evidence has become available that instillation of BCG into the bladder is an effective form of immunotherapy in this disease. While the mechanism is unclear, it appears a local immune reaction is mounted against the tumor. Immunotherapy with BCG prevents recurrence in up to 67% of cases of superficial bladder cancer. Thus, about 30% of patients experience treatment failure, which cannot be predicted in advance and carries a high risk of disease progression.
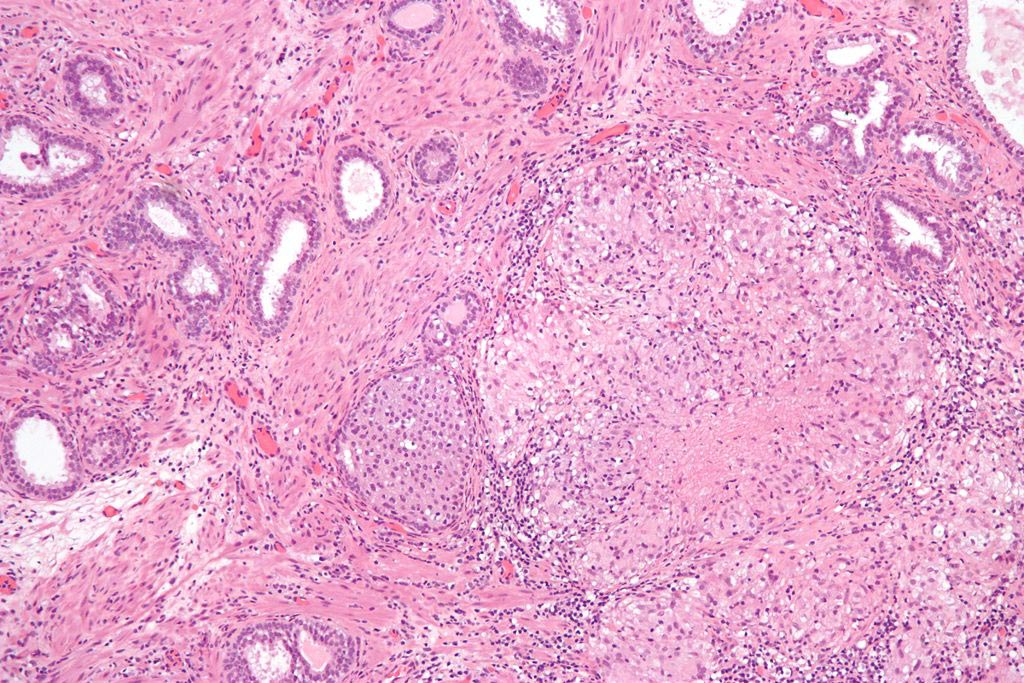
Image: A micrograph showing granulomatous inflammation of bladder neck tissue due to Bacillus Calmette-Guérin (BCG) used to treat bladder cancer (Photo courtesy of Wikimedia Commons).
Investigators at Brighton and Sussex Medical School (United Kingdom) examined the in vitro tuberculin-responsiveness of CD4+ T cells before BCG immunotherapy in 42 patients with high-risk (non-muscle invasive bladder cancer) NMIBC. The frequencies and functionalities of cytokine-expressing CD4+ T cells immediately before and after BCG immunotherapy induction were assessed by flow cytometry after overnight tuberculin stimulation. Tuberculin-induced secreted mediators were measured by electrochemiluminescence. The results were correlated with recurrence-free patient survival six months after induction.
Results revealed that a tuberculin-induced, secreted, IL2 concentration higher than 250 picograms per milliliter was the best predictor of recurrence-free survival, providing 79% sensitivity, 86% specificity, and overall correct classification in 78.6% of cases. In 50% of patients later experiencing recurrence, but not in any of the recurrence-free survivors, IL2 secretion was less than 120 picograms per milliliter.
Senior author Dr. Florian Kern, professor of immunology at Brighton and Sussex Medical School, said, "The simplicity of our new test makes it very attractive as a clinical test. There are several tests for tuberculosis that are ultimately based on the same test principle and have been rolled out across the world in recent times."
The study was published in the August 17, 2018, online edition of the journal Cancer Immunology Research.
Related Links:
Brighton and Sussex Medical School














