DNA Screening and Invasive Testing Show Similar Miscarriage Rates
By LabMedica International staff writers
Posted on 29 Aug 2018
Cell-free DNA (cfDNA) tests are increasingly being offered to women in the first trimester of pregnancies at a high risk of trisomy 21 to decrease the number of required invasive fetal karyotyping procedures and their associated miscarriages.Posted on 29 Aug 2018
The rates of miscarriage following invasive procedures only in the case of positive cfDNA test results have been compared with immediate invasive testing procedures such as amniocentesis or chorionic villus sampling in women with pregnancies at high risk of trisomy 21 as identified by first-trimester combined screening.
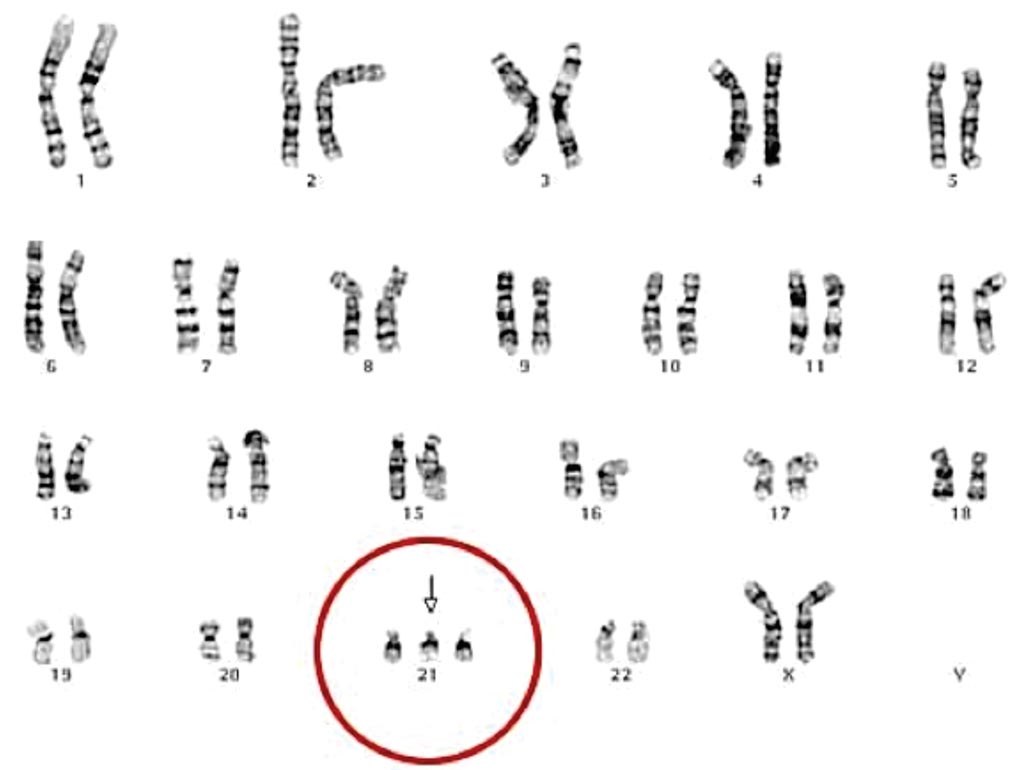
Image: Karyotyping of Trisomy 21 in a female: There is a full set of 23 homologous pairs of autosomes, but an extra chromosome 21 (Photo courtesy of Dr. Daniel Closa).
Scientists collaborating with those at the Hôpital Necker–Enfants Malades (Paris, France) conducted randomized clinical trial from April 8, 2014, to April 7, 2016, in 57 centers in France among 2,111 women with pregnancies with a risk of trisomy 21 between 1 in 5 and 1 in 250 following combined first-trimester screening. Patients were randomized to receive either cfDNA testing followed by invasive testing procedures only when cfDNA tests results were positive (n = 1,034) or to receive immediate invasive testing procedures (n = 1,017). The cfDNA testing was performed using an in-house validated method based on next-generation sequencing.
The team reported that 0.8% of women had miscarriages before reaching 24 weeks gestation. The miscarriages occurred at 19.9 weeks gestation, on average, in both the invasive testing group and the group that received initial cfDNA screening. Even so, they noted that just 8.3% of women from the cfDNA screening arm of the study had gone on to get amniocentesis or chorionic villus sampling, compared to nearly 77% of women in the invasive testing arm of the study. In the cfDNA group, they found all 27 instances of trisomy 21, with a false-positive rate of 5.6%, along with one additional case of chromosomal abnormality. They detected 49 chromosomal abnormalities in the group that underwent invasive testing from the get-go, including 11 non-trisomy 21 chromosomal abnormalities.
The authors concluded that among women with pregnancies at high risk of trisomy 21, offering cfDNA screening, followed by invasive testing if cfDNA test results were positive, compared with invasive testing procedures alone, and did not result in a significant reduction in miscarriage before 24 weeks. The study was published on August 14, 2018, in the Journal of the American Medical Association.
Related Links:
Hôpital Necker–Enfants Malades














