Novel Saliva-Based PCR Assay Detects HIV Antibodies Earlier
By LabMedica International staff writers
Posted on 28 Feb 2018
An oral fluid-based test for human immunodeficiency virus (HIV) shows promise as a population-based screening tool. According to its developers, the test is easier to collect than blood but yields results that are just as reliable.Posted on 28 Feb 2018
In comparison to blood, which poses an infection risk to healthcare workers, oral fluid is not infectious. However, the problem with human saliva is it contains very few anti-HIV antibodies, the markers of HIV infection, and current oral fluid assays do not detect HIV as quickly or efficiently as blood tests.
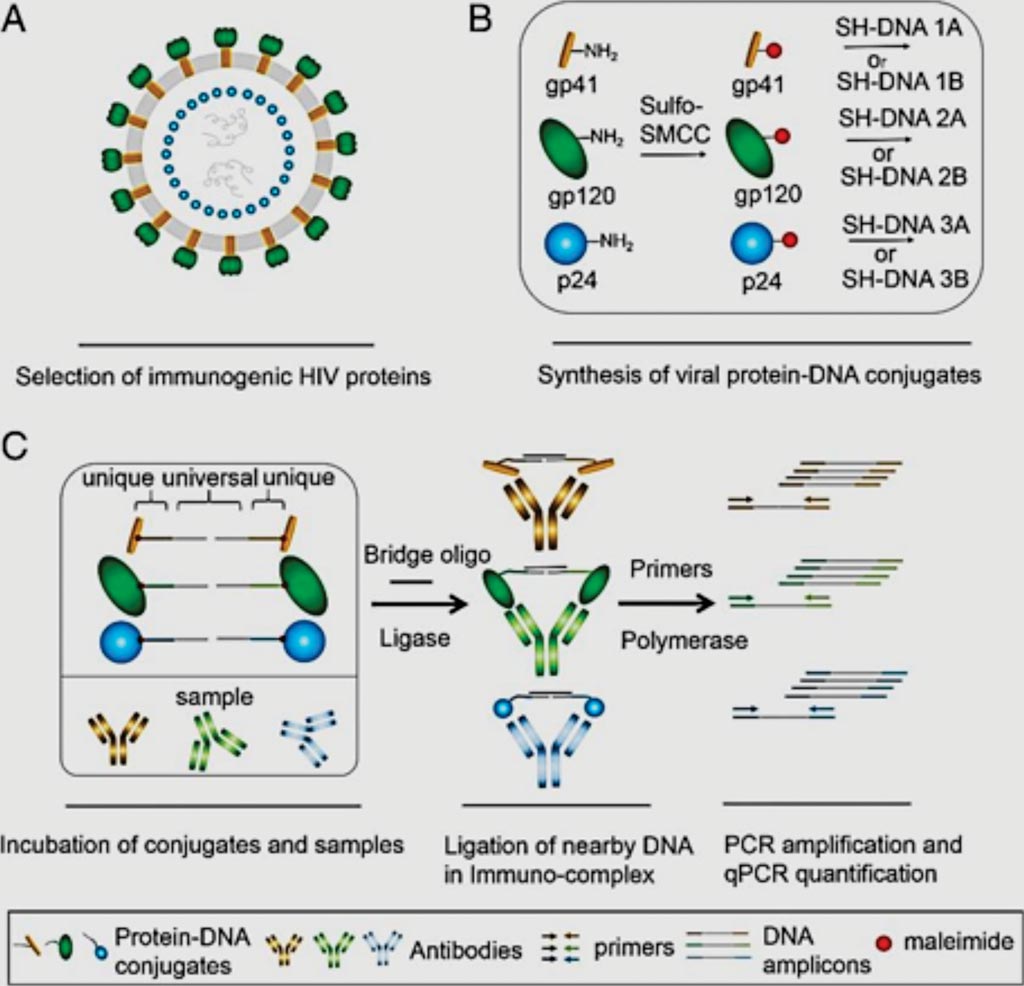
Image: Principle scheme of antibody detection by ADAP for HIV (Photo courtesy of Stanford University).
Scientists at Stanford University (Stanford, CA, USA) have demonstrated how a new oral fluid test, the Antibody Detection by Agglutination– PCR (ADAP) technology, was able to successfully detect HIV at the early stages. The developers of ADAP analyzed how antibodies latch on to the HIV virus to create a more sensitive oral fluid test that was 1,000 to 10,000 times more sensitive than clinical enzyme-linked immunoassays in finding HIV antibodies. ADAP uses polymerase chain reaction (PCR), a technique often used to detect DNA, to find HIV antibodies.
The team used ADAP technology to accurately diagnose 22 individuals who participated in Alameda County Public Health Laboratory’s HIV screening program and had been diagnosed with HIV through other methods. Overall, it yielded 100% clinical sensitivity and 100% specificity for detecting these antibodies from oral fluids. The test also did not produce any false positive results in 22 additional individuals who were HIV negative. The enhanced analytical sensitivity enables this assay to correctly identify HIV-infected individuals otherwise missed by current oral fluid (OF) assays.
Carolyn R. Bertozzi, PhD, a professor of chemistry and the lead investigator of the study, said, “PCR is very sensitive, it can detect very low amounts of the target DNA, whereas techniques people use to detect proteins are far less sensitive. Our test brings the sensitivity of PCR to the testing of proteins, the HIV antibodies in oral fluid, more specifically. We can reset the standards for oral fluid diagnostic sensitivity, bringing it closer to that of blood tests but with convenience of oral fluid. ” The study was published on February 6, 2018, in the journal Proceedings of the National Academy of Sciences.
Related Links:
Stanford University














