Colorectal Cancer Immunohistochemistry Panel Launched
By LabMedica International staff writers
Posted on 13 Feb 2018
The global burden of colorectal cancer is expected to increase by 60% to more than 2.2 million new cases and 1.1 million deaths by 2030. Increasingly, guidelines are recommending universal screening of all newly diagnosed colorectal cancers for Lynch syndrome.Posted on 13 Feb 2018
Lynch syndrome results in a 50% to 80% lifetime risk of developing colorectal cancer, making it important to identify the syndrome in colorectal cancer patients and at-risk family members. Identification of the syndrome may result in early detection and possible cancer prevention among those with the inherited mutation.
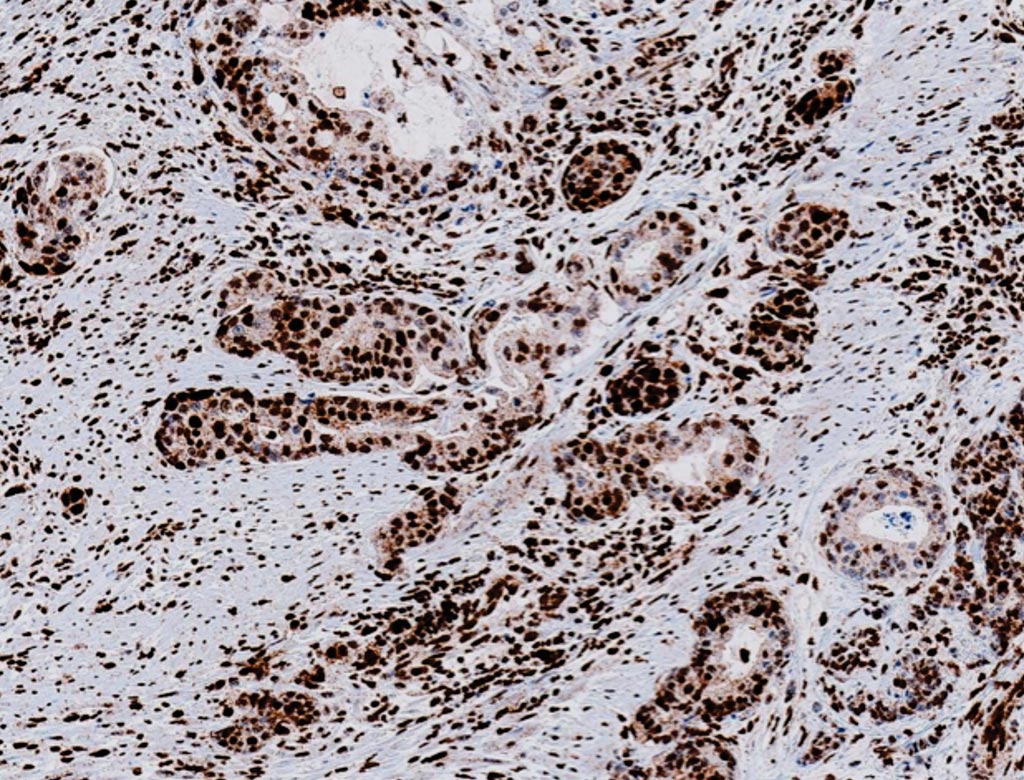
Image: Immunohistochemistry of MLH-1 (M1) Mouse Monoclonal Primary Antibody which is used to qualitatively identify human DNA mismatch repair (MMR) protein MLH1, expressed in the nucleus of normal proliferating cells. Loss of MLH1 is associated with colorectal and other cancers (Photo courtesy of Roche Tissue Diagnostics).
The CE-marked VENTANA MMR IHC Panel (Ventana Medical Systems, Tucson, AZ, USA) has been launched which provides clinicians with a comprehensive group of immunohistochemistry (IHC) tests for patients diagnosed with colorectal cancer. The tests detect certain proteins associated with a DNA repair mechanism called mismatch repair (MMR), and aid in differentiating between sporadic colorectal cancer and probable Lynch syndrome, a hereditary form of colorectal cancer. About 3% of colorectal cancers are associated with Lynch syndrome.
The CE-marked VENTANA MMR IHC Panel consists of VENTANA anti-MLH1 (M1), VENTANA anti-PMS2 (A16-4), VENTANA anti-MSH2 (G219-1129), VENTANA anti-MSH6 (SP93) antibodies, for patients diagnosed with colorectal cancers for the detection of mismatch repair protein deficiency as an aid in the identification of probable Lynch syndrome, and VENTANA BRAF V600E (VE1) antibody as an aid to differentiate between sporadic colorectal cancer and probable Lynch syndrome.
Ann Costello, Head of Roche Tissue Diagnostics, said, “This testing impacts not just the patient, but family members who may benefit from further genetic testing and advanced monitoring to detect colorectal cancer at its earlier stages, when it is more treatable. The VENTANA MMR IHC Panel provides clinicians with an additional tool to perform universal tumor screening for probable Lynch syndrome as recommended by medical guidelines.”













