Analysis Offers Treatment Options for Olfactory Neuroblastoma
By LabMedica International staff writers
Posted on 01 Feb 2018
Olfactory neuroblastoma (ONB) is a rare, locally aggressive, malignant neoplasm originating in the olfactory epithelium in the nasal vault. The recurrence rate of ONB remains high and there are no specific treatment guidelines for recurrent/metastatic ONBs.Posted on 01 Feb 2018
In recent years, with the advancement of molecular diagnostic methods, the focus has been on developing individualized targeted therapies for treating different types of cancer. Cytogenetic studies on ONB revealed diverse and complex genomic imbalances in entire chromosomes and chromosome segments.
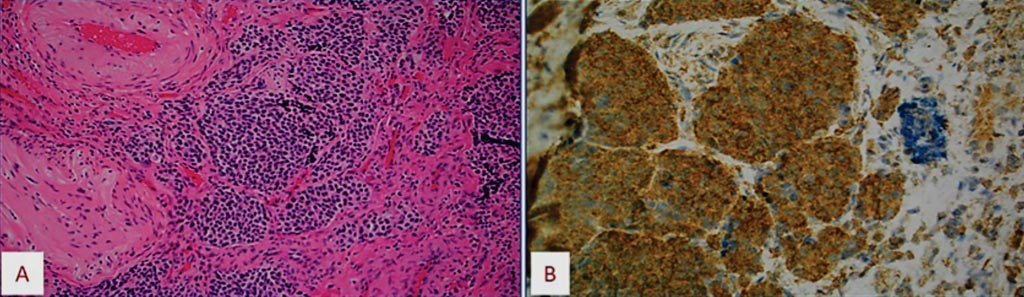
Image: (A) Histology of a case of olfactory neuroblastoma with upregulation of CD24 gene by microarray, (B) confirmed by CD24 protein overexpression in the tumor cells (Photo courtesy of Caris Life Sciences).
Scientists at Caris Life Sciences (Phoenix, AZ, USA) working with their international colleagues retrospectively analyzed 23 formalin-fixed paraffin-embedded (FFPE) samples collected between 2012 and 2017 from 10 male and 13 female olfactory neuroblastoma patients between the ages of 29 and 84 with recurrent or metastatic forms of the disease.
Expression of predictive biomarkers was evaluated immunohistochemically using commercially available antibodies and detection kits by automated staining techniques. Next-generation (NGS) and Sanger sequencing was performed on genomic DNA isolated from 15 FFPE samples using the MiSeq platform. The Illumina TruSeq Amplicon Cancer Panel (TSACP) was used for amplifying specific genomic regions. Six recent ONB cases were tested for gene fusions using the Illumina Archer FusionPlex Solid Tumor Kit. Whole-genome expression (RNA) was analyzed in four samples. The group used fluorescence in situ hybridization or chromogenic in situ hybridization to interrogate copy number patterns for genes such as EGFR, HER2, and cMET.
The team detected mutations in 63% ONBs including TP53, CTNNB1, EGFR, APC, cKIT, cMET, PDGFRA, CDH1, FH, and SMAD4 genes. Twenty-one genes were over-expressed and 19 genes under-expressed by microarray assay. Some of the upregulated genes included CD24, SCG2, and IGFBP-2. None of the cases harbored copy number variations of EGFR, HER2 and cMET genes, and no gene fusions were identified. Multiple protein biomarkers of potential response or resistance to classic chemotherapy drugs were identified, such as cisplatin sensitivity in 10/12 (low ERCC1), irinotecan sensitivity in 12/19 (high TOPO1), vincristine resistance in 13/14 (high TUBB3), and multidrug resistance in 6/6 cases (high MRP1).
Semir Vranić, MD, PhD, an assistant professor of pathology and lead author of the study, said, “We identified multiple protein biomarkers of response or resistance to classic chemotherapy and targeted therapy that could be useful in optimizing the cytotoxic chemotherapy and further improving personalized treatment of olfactory neuroblastoma.” The study was published on January 11, 2018, in the journal Public Library of Science ONE.
Related Links:
Caris Life Sciences













