Droplet Digital PCR Detects Schistosomiasis DNA in Samples
By LabMedica International staff writers
Posted on 08 Nov 2017
Schistosoma japonicum is the only human blood fluke that occurs in China and Philippines and also in Sri Lanka. It is the cause of schistosomiasis japonica, a disease that still remains a significant health problem especially in lake and marshland regions.Posted on 08 Nov 2017
Microscopic identification of eggs in stool or urine is the most practical method for diagnosis. Stool examination should be performed when infection with S. mansoni or S. japonicum is suspected, and urine examination should be performed if S. haematobium is suspected.
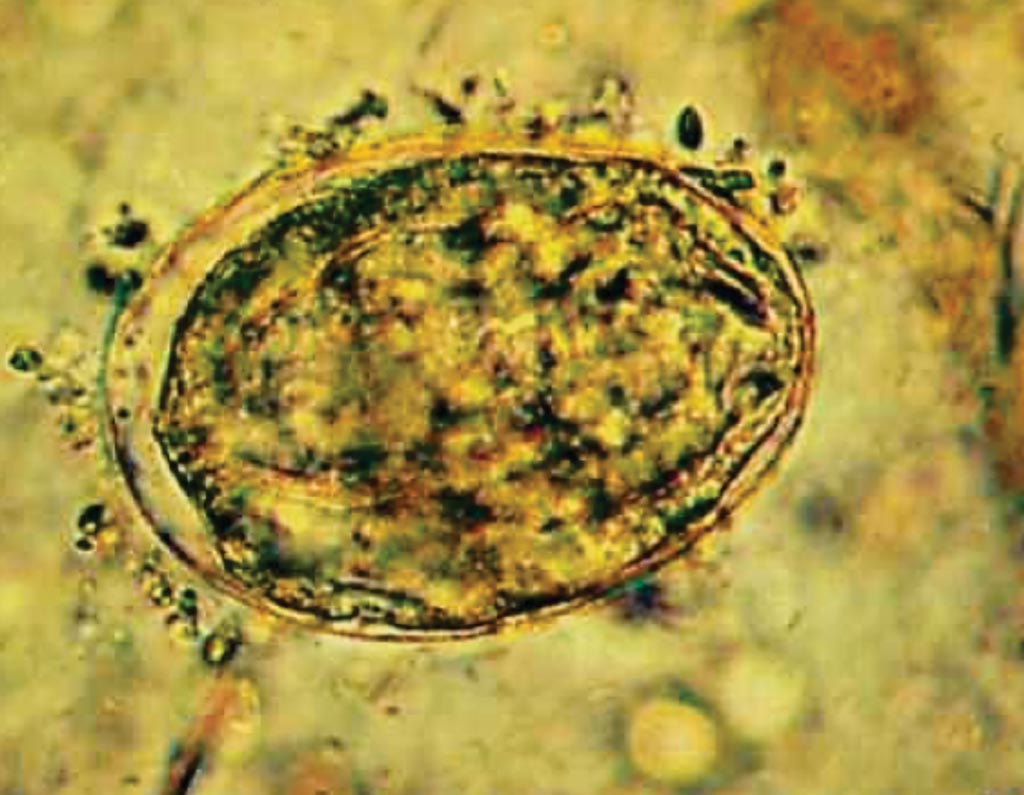
Image: A Schistosoma japonicum egg in feces (unstained, high power). The eggs of this fluke are smaller than those of S. mansoni and S. haematobium. They are ovoid and have a thin, clear shell and a spine or hook-like structure that may be inconspicuous (Photo courtesy of Florida Gulf Coast University).
An international team of scientists led by those at the QIMR Berghofer Medical Research Institute (Brisbane, Australia) have used a previously reported a novel droplet digital-polymerase chain reaction (ddPCR) assay targeting the mitochondrial NADH dehydrogenase subunit I gene (nad1) to diagnose schistosomiasis japonica. The tool identified both pre-patent and patent infections using S. japonicum DNA isolated from serum, urine, salivary glands and feces in a murine model. The assay was validated here using clinical samples collected from 412 subjects resident in an area moderately endemic for schistosomiasis in the Philippines.
The team reported that S. japonicum DNA present in human stool, serum, urine and saliva was detected quantitatively with high sensitivity. The capability to diagnose cases of human schistosomiasis using non-invasively collected clinical samples, the higher level of sensitivity obtained compared with the microscopy-based Kato-Katz test, and the capacity to quantify infection intensity, have important public health implications for schistosomiasis control and programs targeting other neglected tropical diseases.
The authors concluded that the verified ddPCR method represents a valuable new tool for the diagnosis and surveillance of schistosomiasis, particularly in low prevalence and low intensity areas approaching elimination and in monitoring areas where disease emergence or re-emergence is a concern. The study was published on September 27, 2017, in the Journal of Infectious Diseases.
Related Links:
QIMR Berghofer Medical Research Institute







 Analyzer.jpg)





