Liquid Biopsy Panel Yields Complete Molecular Profile
By LabMedica International staff writers
Posted on 06 Sep 2017
The first liquid biopsy pan-cancer panel that simultaneously interrogates exosomal ribonucleic acid (exoRNA) and circulating tumor DNA (ctDNA) in a single assay has been launched.Posted on 06 Sep 2017
The targeted panel for tumor profiling identifies clinically actionable and functionally important mutations across multiple cancer types starting from a small volume of patient blood or plasma.
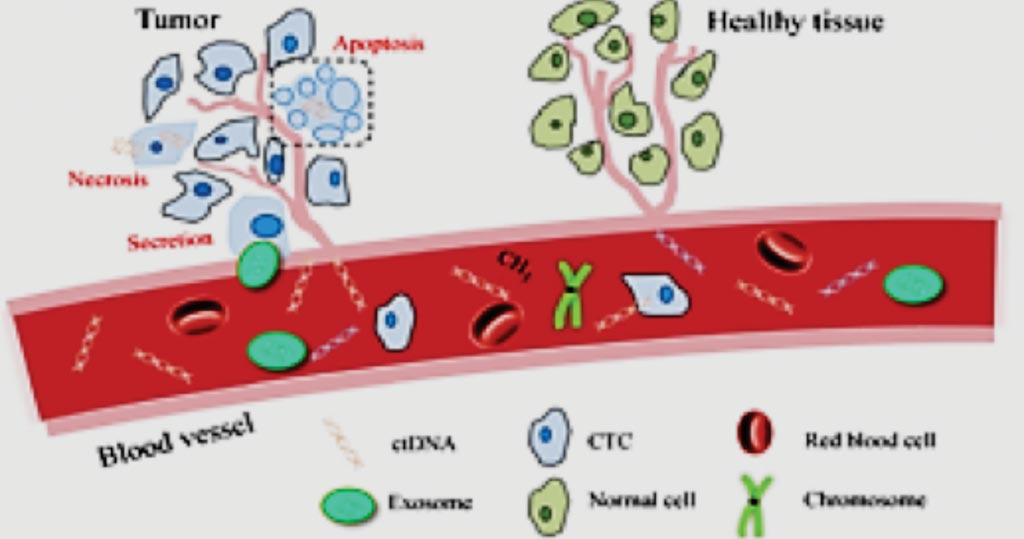
Image: A diagram for the basis of the MedOncAlyzer 170 panel (Photo courtesy of Exosome Diagnostics).
The MedOncAlyzer 170 panel leverages the proprietary Exosome Diagnostics (Waltham, MA, USA) technologies including nucleic acid co-isolation, target capture and bioinformatics to yield unprecedented sensitivity and scope. The primary drivers of ctDNA release into the bloodstream are apoptosis and necrosis of tumor cells. Existing solutions that rely on ctDNA alone are building a profile of the tumor that is biased towards consequences of cell death. Exosomes, in contrast, are actively released by living cells including viable tumor cells.
The MedOncAlyzer 170 panel’s scope includes 170 clinically actionable and functionally relevant gene targets; capture and sequencing of complete coding regions for all targets; 99.9% sensitivity for mutations at allele fractions of equal to or greater than 0.1%; ability to detect RNA-specific events such as gene fusions and splice variants; complete panel coverage from ≥ 0.5mL of the starting material which is plasma; doubles the nucleic acid extracted per mL of sample volume versus ctDNA alone; and 82,000 COSMIC variants interrogated.
Johan Skog, PhD, Chief Science Officer of Exosome Diagnostics, said, “The MedOncAlyzer is the only cancer panel on the market that interrogates information on both RNA and DNA, giving it a higher sensitivity compared to ctDNA assays when profiling early stage and late stage cancers in plasma. The MedOncAlyzer, with its unique ability to co-isolate RNA and DNA, is balanced to produce accurate, highly sensitive identification of rare variants through all stages of disease progression and treatment, including RNA variants that cannot be seen on ctDNA.”
Related Links:
Exosome Diagnostics














