New Assay Facilitates Diagnosis of Rare Infant Disease
By LabMedica International staff writers
Posted on 06 Sep 2017
A novel flow cytometric assay that is easily performed in diagnostic laboratories was developed to improve the ability to diagnose the rare syndrome, hepatic veno-occlusive disease with immunodeficiency (VODI).Posted on 06 Sep 2017
Mutations in the Sp110 (SP110 nuclear body protein) gene are the underlying cause of VODI, a combined immunodeficiency that is difficult to treat and often fatal. VODI is characterized by: (1) primary immunodeficiency; and (2) terminal hepatic lobular vascular occlusion and hepatic fibrosis manifest as hepatomegaly and/or hepatic failure. Onset is usually before age six months. The immunodeficiency comprises severe hypogammaglobulinemia, clinical evidence of T-cell immunodeficiency with normal numbers of circulating T cells, absent lymph node germinal centers, and absent tissue plasma cells. Bacterial and opportunistic infections including Pneumocystis jirovecii infection, mucocutaneous candidiasis, and enteroviral or cytomegalovirus infections occur. In the past the prognosis for affected individuals was poor, with 100% mortality in the first year of life if unrecognized and untreated with intravenous immunoglobulin (IVIG) and Pneumocystis jirovecii prophylaxis.
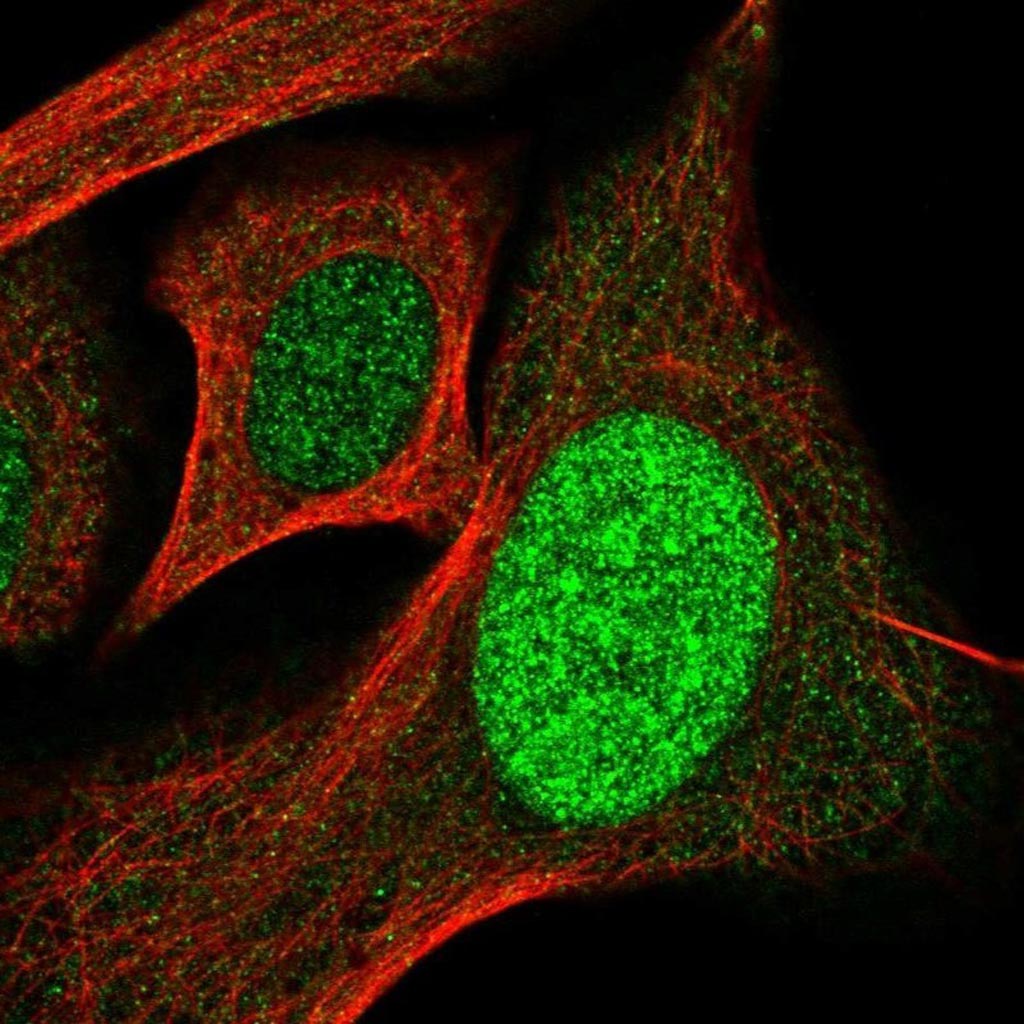
Image: Staining of human cell line U-2 OS with fluorescently labeled SP110 antibody shows localization of the protein to the nucleus (Photo courtesy of Novus Biologicals).
Several factors make establishing the diagnosis of VODI challenging: (1) Current screening strategies to identify severe combined immunodeficiency are based on measuring T-cell receptor excision circles (TREC). This approach will fail to identify VODI patients because the disease is not associated with severe T-cell lymphopenia at birth; (2) the SP110 gene contains 17 exons, making it a challenge for Sanger sequencing. The recently developed next-generation sequencing (NGS) platforms that can rapidly determine the sequence of all 17 exons are available in only a few laboratories; (3) there is no standard functional assay to test for the effects of novel mutations in Sp110; and (4) it has been difficult to use flow cytometry to identify patients who lack Sp110 because of the low level of Sp110 protein in peripheral blood lymphocytes.
To facilitate VODI diagnosis, investigators at the University of Basel (Switzerland) developed a novel flow cytometric assay that is easily performed in diagnostic laboratories and might thus become a standard assay for the evaluation of patients who may have VODI. In addition, the assay will facilitate investigations directed at understanding the function of Sp110.
"We expect that this test will soon be used in laboratories to diagnose a Sp110 deficiency," said senior author Dr. Mike Recher, professor of biomedicine at the University of Basel. "The test will also help us quickly discover more about the biological function of Sp110."
The flow cytometry assay for diagnosis of VODI was described in the August 21, 2017, online edition of the Journal of Clinical Immunology.
Related Links:
University of Basel














