Genetic Mutation Identified for Childhood Kidney Cancer
By LabMedica International staff writers
Posted on 13 Jun 2017
The underlying mechanism that causes aneuploidy might be important in understanding cancer risk and not solely having the wrong number of chromosomes in a cell. It is an important development that provides valuable information about the fundamental biology of cancer.Posted on 13 Jun 2017
Wilms tumor is a form of kidney cancer that occurs mainly in children and it affects about 1 in 10,000 children, but fortunately is curable in about 90% of cases. Individuals with mosaic variegated aneuploidy (MVA) have cells with the wrong number of chromosomes while some have too many, others have too few and this is known as aneuploidy.
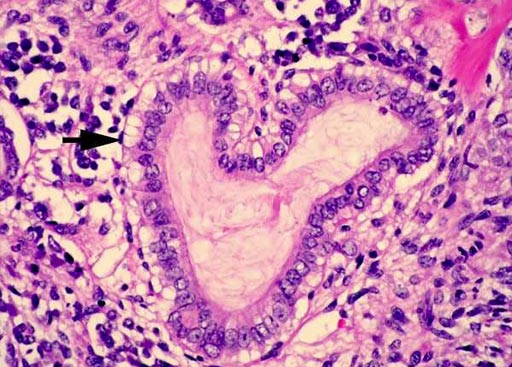
Image: A histopathology of Wilms tumor: the epithelial tubular structures may show mucinous differentiation as indicated. Ciliated epithelium and squamous metaplasia may also be seen (Photo courtesy of Pathpedia).
A large team of scientists collaborating with those at The Institute of Cancer Research (London, UK) investigated 20 families with MVA and their genes were analyzed using a technique called exome sequencing. Mutations in a gene called Thyroid Hormone Receptor Interactor 13 (TRIP13) were found. The team identified six individuals with biallelic loss-of-function mutations in TRIP13. All six developed Wilms tumor. Constitutional mosaic aneuploidies, microcephaly, developmental delay and seizures, which are features of MVA syndrome, were more variably present.
The scientists found that TRIP13-mutant patient cells have no detectable TRIP13 and have substantial impairment of the spindle assembly checkpoint (SAC), leading to a high rate of chromosome missegregation. The study also found that not all children with MVA were at high risk. Those with the TRIP13 mutation, and another previously identified mutation called Mitotic Checkpoint Serine/Threonine Kinase B (BUB1B), were at high risk, but children with MVA due to other causes were not.
Nazneen Rahman, PhD, a professor and senior author of the study, said, “This study has been of immediate use to families in providing a reason for why their child developed cancer, and information about risks to other children, which is very rewarding. Equally importantly the study has provided new information about how aneuploidy and cancer are linked, a topic that has been hotly debated and intensively researched for many decades.” The study was published on May 29, 2017, in the journal Nature Genetics.
Related Links:
The Institute of Cancer Research














