LAMP-Kit Diagnoses Non-Falciparum Malaria
By LabMedica International staff writers
Posted on 26 Jan 2017
Malaria incidence has recently been declining globally, although in many African countries it is still one of the top health problems and the efforts to control and eradicate malaria will still need a strong diagnostic capacity, which allows any parasitaemic patient to be detected and treated sooner.Posted on 26 Jan 2017
Loop-mediated isothermal amplification (LAMP) is a simple molecular diagnostic method based on the principle of isothermal amplification, which does not require special equipment or special distribution in laboratories, and provide results in 60 minutes. A LAMP-kit can be used in the field without special equipment and could have an important role in malaria control programs in endemic areas and for malaria diagnosis in returned travelers.
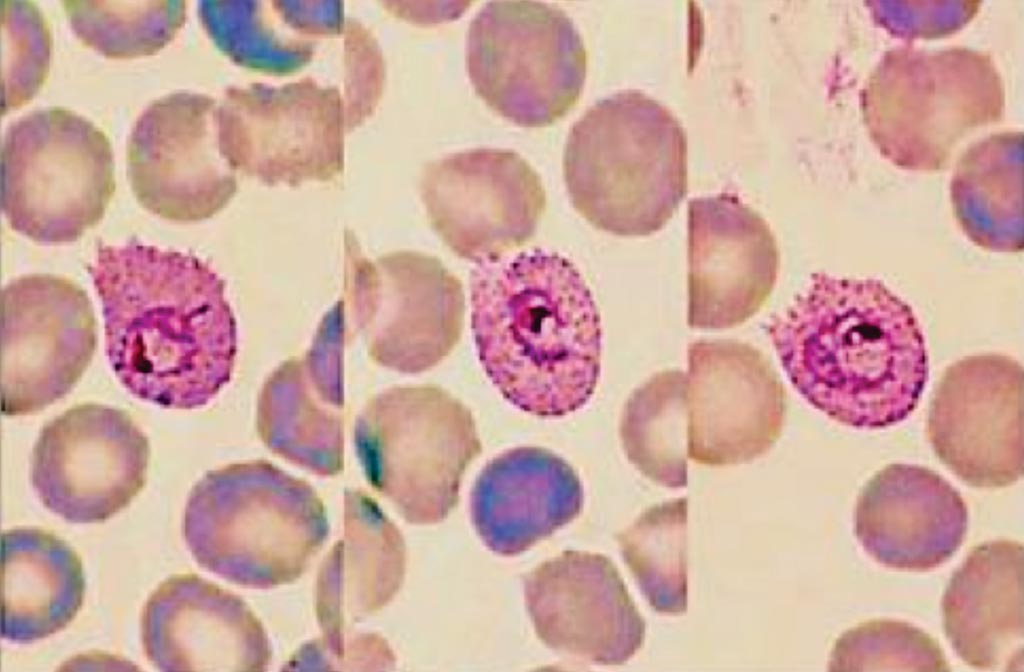
Image: Three typical trophozoites of Plasmodium ovale in a thin blood smear; note the fimbriation and Schüffner\'s dots (Photo courtesy of the Centre for Agriculture and Bioscience International).
Scientists at the National Center for Microbiology of Majadahonda and their colleagues have determined the diagnostic validity of a LAMP-kit to diagnose Plasmodium ovale and compare it to microscopy and conventional multiplex nested polymerase chain reaction (nPCR) in the diagnosis of P. ovale infections in archived clinical specimens. They also compared the naked eye reading of the amplification products with the automated reading by turbidimetry as well as to determine the specificity of the P. falciparum LoopAmp primer in specimens of patients infected with P. ovale.
Plasmodium ovale positive samples (29, tested by PCR and/or microscopy) and malaria negative specimens (398, tested by microscopy and PCR) were collected in different hospitals of Europe from June 2014 to March 2016. DNA was extracted from all samples and amplification was performed with LoopAMP MALARIA kit in an Eiken 500 automated turbidimeter. The results of LAMP read by turbidimetry and with the naked eye were compared.
The LAMP-kit showed a sensitivity of 100% and a specificity of 97.24% with positive and negative predictive values of 72.5% and 100%, respectively. Naked eyed readings were in accordance with turbidimetry readings (sensitivity, 92.5%, specificity, 98.96% and positive and negative predictive values, respectively, 90.24% and 99.22%). The limit of detection of LAMP assay for P. ovale was between 0.8 and 2 parasites/µL. P. falciparum LAMP reactions were found to be negative in 29 out of 29 confirmed P. ovale samples. The one P. ovale sample positive by P. falciparum LAMP was excluded as it was found to be also positive for P. falciparum by nPCR.
The authors concluded that the Pan primer of the Malaria kit LoopAMP can detect P. ovale at very low-levels and it showed a predictive negative value of 100%. The relative simplicity of the LAMP procedure and the low infrastructure costs open a range of opportunities by bringing molecular-level parasite detection and capacity of using malaria LAMP in field settings. The study was published on January 7, 2017, in the Malaria Journal.









 (3) (1).png)




