First-Ever Gene Identified with Biological Origin of Schizophrenia
By LabMedica International staff writers
Posted on 07 Feb 2016
In a landmark study, researchers have identified mutants of the complement component 4 (C4) gene that are strongly associated with the risk of developing schizophrenia. Posted on 07 Feb 2016
They further discovered that C4, the well-known immune system protein, is also involved in “synaptic pruning” in the brain. The study provides important insights into the biological origin and underlying physiology of this debilitating disease, and likely a new path for developing early-detection diagnostic tests as well as more effective drugs for treatment and prevention—by allowing therapeutic strategies to be directed at the disorder’s roots, rather than its symptoms.
“Since schizophrenia was first described over a century ago, its underlying biology has been a black box, in part because it has been virtually impossible to model the disorder in cells or animals,” said study leader Steven McCarroll, associate professor at Harvard Medical School and director of genetics for the Stanley Center for Psychiatric Research at the Broad Institute (Cambridge, MA, USA), “The human genome is providing a powerful new way in to this disease.”
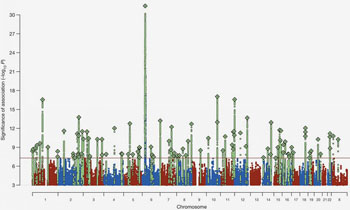
Previously, over 100 regions in the human genome were identified that carry risk factors for schizophrenia. The strongest risk-association signal was by far on chromosome 6, in a region long associated with infectious disease, causing some to suggest that schizophrenia might be triggered by an infectious agent. Schizophrenia’s strongest known genetic association at a population level involves variation in the immune-system’s major histocompatibility complex (MHC) locus, but the genes and molecular mechanisms accounting for this have remained elusive using conventional genetic methods. The new study shows that this association arises in part from many diverse alleles of the C4 genes. By examining RNA levels, the researchers found that these alleles generated widely varying C4A and C4B expression levels in the brain.
The study was based on DNA collected from over 100,000 people from 30 countries, detailed analysis of complex genetic variation in more than 65,000 human genomes, development of an innovative analytical strategy, examination of postmortem brain samples from hundreds of people, and the use of animal models to further study biological function of C4.
Analysis of genome data of nearly 65,000 people (with and without schizophrenia) revealed a striking correlation: patients who had particular C4 alleles showed higher expression of that allele, as well as higher risk of developing schizophrenia. So mutations leading to abnormal overexpression of C4 were strongly associated with predisposition to schizophrenia.
How does C4—known to mark infectious microbes for destruction by immune cells—affect the risk of schizophrenia? The researchers found that human C4 protein localized to neuronal synapses, dendrites, axons, and cell bodies. They also discovered that C4 plays a key role in pruning synapses during brain maturation. In particular, C4 was necessary for complement component 3 (C3) to be deposited onto synapses, as a tag signaling that those synapses should be pruned. The data also suggested that higher C4 activity is associated with increased elimination of brain synapses at a key period in brain development. In mice, C4 mediated synapse elimination during postnatal development.
The human brain normally undergoes widespread synapse pruning (especially in the cerebral cortex) during adolescence, the typical period of symptoms onset. “Normally, pruning gets rid of excess connections we no longer need, streamlining our brain for optimal performance, but too much pruning can impair mental function,” explained Thomas Lehner, PhD, director, Office of Genomics Research Coordination of the NIH’s National Institute of Mental Health (NIMH; Bethesda, MD, USA), “It could help explain schizophrenia’s delayed age-of-onset of symptoms in late adolescence/early adulthood and shrinkage of the brain’s working tissue.” The findings may also help explain the longstanding mystery of why brains from people with schizophrenia tend to have a thinner cerebral cortex with fewer synapses than unaffected individuals.
The results revealed that excessive pruning of brain synapse connections predisposes to schizophrenia. The fortunate fact that much is already known about the role of C4 and other complement proteins in the immune system means that researchers can tap into a wealth of existing knowledge to identify possible therapeutic approaches. For example, anti-complement drugs are already under development for treating other diseases.
“While it’s still early days, we’ve seen the power of understanding the biological mechanism of disease in other settings,” said Eric Lander, director of the Broad Institute, “Understanding schizophrenia will similarly accelerate progress against this devastating disease that strikes young people.”
The work was enabled in large part by catalytic funding from the Stanley Center and the paper has been dedicated to the late Ted Stanley. “Through philanthropy, we have been able to take bets on risky science with potentially transformative results,” said Stanley Center director Steven Hyman, “With support from the late Ted and Vada Stanley, Broad scientists have the freedom to bring together people, capabilities, and resources in innovative ways.”
“This study marks a crucial turning point in the fight against mental illness. It changes the game,” added Bruce Cuthbert, PhD, acting NIMH director, “Thanks to this genetic breakthrough we can finally see the potential for clinical tests, early detection, new treatments, and even prevention.”
The study, by Sekar A et al., was published online ahead of print November 27, 2016, in the journal Nature.
Related Links:
Broad Institute
[US] National Institute of Mental Health (NIMH)
More about the study