Cancer DNA in Blood Helps Personalize Liver Cancer Treatment
By LabMedica International staff writers
Posted on 16 Nov 2015
Liver cancer tumor specimens are difficult and sometimes dangerous to obtain, and noninvasive methods are required to assess cancer progression and characterize underlying genomic features.Posted on 16 Nov 2015
The detection of DNA released by damaged cancer cells, called circulating tumor DNA (ctDNA), in serum before surgery could predict the recurrence of cancer and its metastatic spread through the body in patients with an advanced form of the most common type of liver cancer.
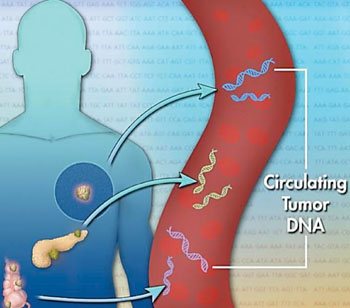
Image: Schematic diagram of circulating tumor DNA (ctDNA), a small piece of DNA released from dying cancer cells into the blood (Photo courtesy of Jonathan Bailey).
Scientists at the Hiroshima University (Japan) and their colleagues analyzed 46 patients with hepatocellular carcinoma who underwent hepatectomy or liver transplantation and for whom whole-genome sequencing data was available. They designed personalized assays targeting somatic rearrangements of each tumor to quantify serum ctDNA. Exome sequencing was performed using cell-free DNA paired primary tumor tissue DNA from a patient with recurrent liver cancer after transcatheter arterial chemoembolization (TACE).
The team used a chemiluminescent immunoassay (Fujirebio; Tokyo, Japan) and chemiluminescent enzyme immunoassay (Abbott Laboratories, Abbott Park, IL, USA) to analyze α-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP), respectively. Thresholds for AFP and DCP abnormalities were defined as 10 ng/mL and 30 mAU/mL, respectively. Cell-free DNA was extracted from 100 μL of preoperative serum by the SMI-TEST (Genome Science Laboratories, Tokyo, Japan). Real-time polymerase chain reaction (PCR) analysis was performed on samples that tested positive in the detection assay and for which serial serum samples were available. PCR was performed using the Mx300P System (Applied Biosystems, Foster City, CA, USA).
The investigators were able to identify 25 common mutations in samples of cell-free DNA, which includes DNA from both normal cells and cancer cells, and DNA from tumors themselves. Furthermore, 83% of mutations identified in the tumor tissues could be detected in the cell-free DNA. They found ctDNA in seven patients and they say that ctDNA has the potential to be a noninvasive way of studying the genetic rearrangements that a cancer has undergone. This information could help doctors provide targeted therapy specific to a patient's cancer. They also demonstrated that the level of serum ctDNA reflected the treatment effect and the progression of hepatocellular carcinoma (HCC). The study was published in the September 2015 issue of the journal Cellular and Molecular Gastroenterology and Hepatology.
Related Links:
Hiroshima University
Fujirebio
Abbott Laboratories