Four Susceptibility Loci Identified for Testicular Germ Cell Tumor
By LabMedica International staff writers
Posted on 09 Nov 2015
Testicular germ cell tumor (TGCT) is the most common cancer in men aged 15 to 45 years, with over 18,000 new cases diagnosed annually in Europe and the incidence of TGCT has approximately doubled over the last four decades in Western Europe, which implicates environmental or lifestyle factors as risk determinants.Posted on 09 Nov 2015
There are no current treatment options that target specific genetic mutations in testicular cancer, and standard treatment with platinum-based chemotherapy has a high success rate. That means that the more likely application of genetic testing in testicular cancer is in diagnosing the level of risk in men yet to develop it, rather than in matching patients to specific treatments.
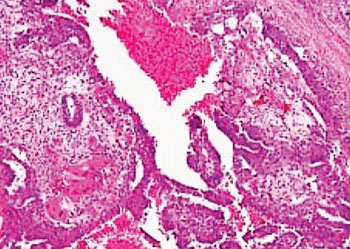
Image: Histopathology of a testicular germ cell tumor consisting of teratoma, embryonal carcinoma yolk sac tumor and syncytiotrophoblasts (Photo courtesy of Lee Moffitt Cancer Center).
Scientists at the Institute of Cancer Research (ICR; London, UK) and their colleagues that carried out a study of more than 25,000 men has uncovered four new genetic variants associated with increased risk of testicular cancer. They discovered four new variants through analyzing the DNA of 6,059 patients with testicular cancer, and comparing it with the DNA of 19,094 people without the disease.
Genotyping for stages 1 and 2 was performed and, stage 1 cases were genotyped on the Illumina HumanCNV370-Duo bead array (Illumina; San Diego, CA, USA) and controls were genotyped on the Illumina Infinium 1.2M array. They used data on 314,861 single nucleotide polymorphism (SNPs) that were successfully genotyped on both the arrays. Stage 2 genotyping was conducted using a custom Illumina Infinium array (iCOGS array) comprising 211,155 SNPs.
These variants, combined with all 21 previously characterized using genetic sequencing, identified men with a 10-fold higher risk of testicular cancer than the population average. Carrying two copies of a single-letter change in the DNA of chromosome 16 led to a 35% increase in activity of the gene glutathione S-transferase pi (GSPT1) in men with testicular cancer, compared with those without. This gene has a role in controlling cell division and has been shown to have increased activity in cancers of the breast, stomach and prostate.
Clare Turnbull, PhD, a senior author of the study said, “Our study identified four new genetic risk factors for testicular cancer. Through previous studies, many led by our team at the ICR, this brings the total number of genetic variants known to be associated with testicular cancer to 25. Applying these 25 variants, we found that men in the top 1% for testicular cancer risk were at a more than ten-fold elevated risk of developing the disease compared with the average—although that still adds up to only around a 5% chance of developing testicular cancer.” The study was published on October 27, 2015, in the journal Nature Communications.
Related Links:
Institute of Cancer Research
Illumina














