Genetic Test Would Help Cut Bowel Cancer Spread
By LabMedica International staff writers
Posted on 15 Oct 2014
Screening families of patients with bowel cancer for a genetic condition would cut their risk of developing bowel, womb, and ovarian cancers.Posted on 15 Oct 2014
Lynch syndrome (LS) is an inherited autosomal dominant disorder characterized by an increased risk of colorectal cancer (CRC) and other cancers, and caused by mutations in the DNA mismatch repair genes.
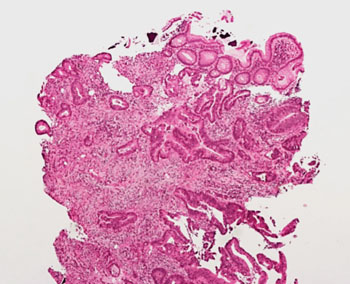
Image: Histopathology of non-polyposis colorectal cancer or Lynch Syndrome (Photo courtesy of Dr. L. Marcucci MD).
Scientists at the University of Exeter Medical School (UK) and Cardiff University (UK) evaluated the accuracy and cost-effectiveness of strategies to identify LS in newly diagnosed early-onset CRC patients who were less than 50 years of age. Cascade testing of relatives is employed in all strategies for individuals in whom LS was identified. The investigators conducted systematic reviews of the test accuracy of microsatellite instability (MSI) testing or immunohistochemistry (IHC) in individuals with CRC at risk of LS, and of economic evidence relating to diagnostic strategies for LS.
The team identified and assessed 42 studies in total, before constructing a computer model of screening strategies for Lynch Syndrome. These were of mixed quality, with significant methodological concerns identified for most. IHC and MSI can both play a part in diagnosing LS but neither is gold standard. The maximum health benefit to the population of interest would be obtained using universal germline testing, but this would not be cost-effective.
The findings indicate that screening the 1,700 people under the age of 50 who are newly diagnosed with bowel cancer each year would identify two thirds of these whose cancer was caused by Lynch Syndrome. From this group, the findings suggest that 40 further cases of cancer could be avoided in them and their relatives. The team used the same model to estimate that 28 cancer related deaths, 24 from bowel cancer and four from womb cancer, could be prevented each year if Lynch Syndrome screening for people with bowel cancer was introduced.
Ian Frayling, PhD, MRCPath, a senior coauthor, said, “If Lynch Syndrome is identified as the cause of bowel cancer, patients can be offered risk-reducing measures such as more intensive postoperative colonoscopy surveillance to spot recurrences and new cancers early. As close relatives have a 50% chance of sharing the gene, screening would provide a valuable opportunity to detect the condition in children, siblings, parents and more distant relatives. It would mean measures could be taken to reduce the risk of cancers developing.” The study was published in the September 2014 issue of the journal Health Technology Assessment.
Related Links:
University of Exeter Medical School
Cardiff University