Molecular Test Finds Increased Chikungunya Virus Infections
By LabMedica International staff writers
Posted on 24 Sep 2014
Chikungunya is an infectious disease that causes fever, rash, and for a subset of patients, chronic joint pain. Pregnant women who acquire Chikungunya infection within one week of delivery can transmit the virus to their baby, which can lead to severe infection.Posted on 24 Sep 2014
Clinical laboratory testing for Chikungunya virus may involve molecular reverse transcription polymerase chain reaction (RT-PCR), which identifies the ribonucleic acid (RNA) of the virus and immunoassays, which assess blood-serum levels of the antibodies Immunoglobulin M and G (IgM and IgG).
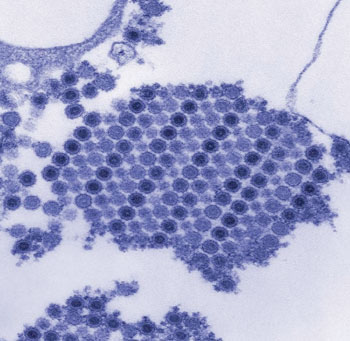
Image: Digitally-colorized transmission electron micrograph (TEM) depicts numerous Chikungunya virus particles, which are composed of a central dense core that is surrounded by a viral envelope. Each virion is approximately 50 nm in diameter (Photo courtesy of Cynthia Goldsmith).
Medial experts from Focus Diagnostics (Cypress, CA, USA) analyzed de-identified clinical results of RT-PCR and IgG/IgM tests for Chikungunya ordered by clinicians between January 1 and August 2, 2014. The investigators determined that 2,947 patient samples were tested for antibodies to the virus by Focus Diagnostics clinical laboratory between January 1 and August 2, 2014. Of this total, 82% (2,402) were tested in June and July, suggesting a surge in test volume over the summer. Another 589 RT-PCR tests were also performed on patients. Eighty-eight specimens tested by both methods were ordered and performed.
About 22% of the total tested for antibodies, were IgG and/or IgM positive, suggesting a diagnosis of Chikungunya. Most patients underwent antibody testing well after initial infection. Sixteen percent of specimens exhibited an IgG positive/IgM positive antibody pattern, compared to 5% of specimens that were IgG negative/IgM positive. In addition, the rate of IgG/IgM positive specimens increased since June. These findings suggest that most patients were tested well after initial infection with the virus.
The investigators also determined that of 589 specimens tested by RT-PCR, 168 (28%) were positive for the Chikungunya virus. All positive PCR tests were performed during or after April 2014, possibly because of increased awareness of Chikungunya infection and PCR's role in diagnosing early infection. RT-PCR is helpful for detecting the virus within the first week of infection, but less reliably afterwards.
Hollis Batterman, MD, the medical director at Focus Diagnostics, said, “Our findings suggest that PCR and antibody testing should be considered in anyone with a compatible clinical syndrome who has traveled to or lives in areas with the species of mosquitos that carry the virus. The majority of seropositive samples were IgG/IgM positive, suggesting that most patients were tested later in the onset of infection. In the absence of PCR testing, antibody IgG/IgM patterns may be useful to infer onset of illness, and potential risk of transmission if bitten by mosquitoes.” The study was presented at the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy held September 5–9, 2014, in Washington DC (USA).
Related Links:
Focus Diagnostic













