Proteomic Profiling Enables Earlier Diagnosis of Pancreatic Cancer
By LabMedica International staff writers
Posted on 02 Apr 2014
A newly developed method has correctly identified pancreatic cancer potential with higher accuracy than cytology and cyst fluid carcinoembryonic antigen (CEA) testing, and could be used to improve prevention and reduce unwarranted surgery.Posted on 02 Apr 2014
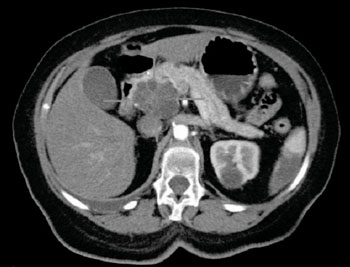
Current prognosis for pancreatic cancer (PCa) is such that only about 5% of patients survive 5 years after diagnosis. Pancreatic cystic lesions (PCLs) almost invariably reflect an underlying inflammatory or neoplastic condition, ranking among the most important incidentalomas to have emerged with radiological advances. This offers an opportunity for preventive intervention against pancreatic cancer (ductal adenocarcinoma) because a substantial proportion of cystic tumors can be considered precursor lesions – of malignant potential (premalignant or malignant tumors). Although PCLs (found in 10% of the population above age 70 years, and common also in younger people) can now be discovered with CT or MRI, imaging alone cannot determine which cysts are at risk for developing into cancer. It often becomes necessary to puncture the cyst and test for tumor markers in the fluid; however these analyses are not reliable. Removing the cyst by surgery, knowing that it may turn out to be benign is also problematic, since the operation is extensive with considerable risks for the patient.
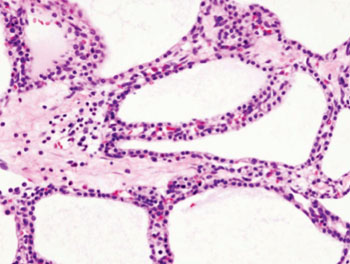
Much better diagnostic tools are required for patients to benefit from PCL detection. Researchers at The Sahlgrenska Academy (Gothenburg, Sweden) have now developed a mass spectrometry (MS) based method that identified PCa precursor cysts with 97% certainty (of 79 examined cysts). The method detects the presence of mucus protein, mucins, in the cystic fluid. The PCL proteomic mucin profiling accuracy was nearly identical (96.6% vs. 98.0%) between the discovery (n = 29) and validation (n = 50) cohorts. “This is an exceptionally good result for a diagnostic test, and we are very hopeful that the method will enable more instances of early discovery of pancreatic cancer, at a stage when the cancer can be treated or prevented,” said Dr. Karolina Jabbar, PhD student at The Sahlgrenska Academy and physician at The Sahlgrenska University Hospital.
The method was also tested in a blinded analysis of existing tumors and determined with about 90% certainty which tumors have already developed into cancers. Therefore, the method could also be used to determine which patients require immediate surgery and which can wait while under continued monitoring.
Professor Gunnar C. Hansson, who initiated the study with senior physician and assistant professor Riadh Sadik, is convinced that MS-based proteomics will soon be introduced in health care: “The technique has been developed, and we can now measure biomarkers both quickly and exactly. Moreover, the method requires minimal biomaterial, in this case 25 times less cyst fluid than conventional tumor marker analyses. I am certain that within 5 years the mass spectrometers will have moved into the hospital corridors”, he said.
Jabber et al. describe the method in the Journal of the National Cancer Institute, February, 2014.
Related Links:
The Sahlgrenska Academy