New Tool Developed to Identify Genetic Risk Factors
By LabMedica International staff writers
Posted on 13 Feb 2014
A new biological pathway-based computational model has been developed to identify underlying genetic connections between different diseases.Posted on 13 Feb 2014
The model called the Pathway-based Human Phenotype Network (PHPN) mines the data present in large publicly available disease datasets to find shared single nucleotide polymorphisms (SNPs), genes, or pathways and expresses them in a visual form.
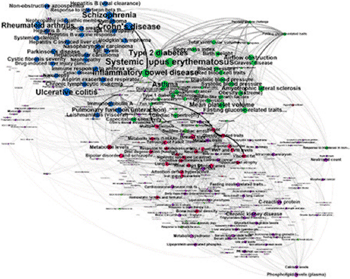
Image: Pathway-based Human Phenotype Network classification shows relationships between diseases and traits based on shared etiology for certain phenotypes. The diseases and traits in these clusters have more connections to each other than to others in the network: the bolder the line, the stronger the connection (Photo courtesy of Dartmouth College).
Geneticists at the Geisel School of Medicine at Dartmouth (Hanover, NH, USA) built a pathway-based human phenotype network (PHPN) of over 800 physical attributes, diseases, and behavioral traits, based on about 2,300 genes and 1,200 biological pathways. Using genome-wide association study (GWAS) phenotype-to-genes associations, and pathway data from a free, open-source, curated and peer reviewed pathway database Reactome, they connected human traits based on the common patterns of human biological pathways, detecting more pleiotropic effects, and expanding previous studies from a gene-centric approach to that of shared cell-processes.
PHPN supports the integration of genomic and phenotypic data to uncover significant links between traits, attributes, and disease. This offers tremendous potential in identifying risk factors for certain diseases. At the same time, it can reveal important targets for therapeutic intervention. The automatic classification of phenotypes into “phenotype classes,” using the network’s topological modularity and a standard community detection algorithm, showed very promising results. Two traits that were connected in the PHPN but did not share any common associated genes or any clear-cut biological relationship were von Willebrand factor and FVIII levels (vWF) and hippocampal atrophy (HA).
Christian Darabos, PhD, the lead author of the study, said, “The intuitive network representation of the knowledge mined from several large-scale datasets makes the information accessible to anyone. It lies at the crossroads of computational genetics, systems biology, information theory, and network science. As a proof of concept, the PHPN has proven capable of identifying well-documented interactions, and many novel links that remain to be explored in depth. The PHPN is a hypothesis-generating tool, potentially capable of identifying yet uncharacterized common drug targets.” As a next step, the scientists will refine statistical methods, isolate networks for optimal results, and compare previous work on phenotype networks. The study was published on January 25, 2014, in the journal BioDataMining.
Related Links:
Geisel School of Medicine at Dartmouth
Reactome













