Next-Generation Breast Cancer Tests Provide More Precise Prognosis
By LabMedica International staff writers
Posted on 12 Feb 2014
Two next-generation genomics breast-cancer tests that determine molecular subtypes and risk of recurrence were highlighted at the 2014 Personalized Medicine Conference.Posted on 12 Feb 2014
The "BluePrint" molecular diagnostics assay from Agendia (Irvine, CA, USA) is the most widely available test providing molecular subtyping of individual breast cancers. It is performed as part of Agendia's "Symphony", the predictive, multigene breast cancer testing panel that also includes "MammaPrint", the first FDA-cleared test of its kind (an IVDMIA breast cancer recurrence assay) and the only one providing risk recurrence information based on prospective trials including patient outcome data (e.g., the RASTER study). MammaPrint provides definitive high-risk or low-risk information about breast cancer recurrence, without ambiguous “intermediate” results.
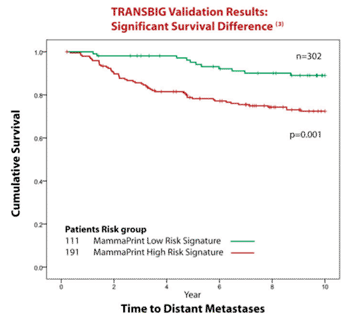
Image: TRANSBIG – translational breast-cancer research validation results for MammaPrint (Photo courtesy of Agendia).
The BluePrint test, building on the foundational prognostic precision of MammaPrint, classifies breast cancer into one of four molecular subtypes: Luminal A, Luminal B, HER2-type, and Basal-type. BluePrint also provides information about neoadjuvant chemosensitivity (responsiveness to chemotherapy) more accurately than does an IHC/FISH assessment. Together, these tests help physicians determine a patient’s individual risk for metastasis, and so which patients can instead be treated with less arduous, and less costly, therapies.
Molecular subtyping provides a more precise prognosis and valuable guidance about the best treatment for early-stage breast cancer, according to the presentation by Neil Barth, MD, a medical oncologist and chief medical officer of Agendia, and a featured speaker at the Personalized Medicine World Conference, held January 27–28, 2014, in Mountain View (CA, USA), with over a thousand clinical and industry participants.
Dr. Barth’s talk outlined how BluePrint and MammaPrint greatly improve the physician’s ability to personalize treatment to the specific biology of each breast cancer. “Molecular subtyping provides us more information about each individual patient’s breast cancer than is available from traditional biomarkers,” said Dr. Barth. “By combining the information from BluePrint and MammaPrint, we can better predict the benefits of therapy. This means we can personalize treatment and in some cases confidently assure patients they can avoid chemotherapy, and the side-effects that go with it, because other therapies will be more effective.” Also, in some cases where the initial recommendation is to treat with chemotherapy and radiation, a patient may give serious consideration to having a double prophylactic mastectomy. If the MammaPrint and BluePrint test results show she has a molecular subtype of breast cancer that has a low risk of recurrence (e.g., subtype Luminal A), she would not need to undergo mastectomy and in most cases not even chemotherapy.
Agendia's Symphony suite of diagnostic tests was developed using unbiased gene selection, analyzing the complete human genome, to help ensure definitive results for patients. Symphony also includes "TargetPrint,” an ER/PR/HER2 expression assay.
Related Links:
Agendia
Agendia Symphony Panel













