Molecular Basis of Alzheimer’s Disease Determined
By LabMedica International staff writers
Posted on 02 Oct 2013
Alzheimer's disease is thought to be caused by the buildup of abnormal, thread-like protein deposits in the brain, known as beta-amyloid fibrils. Posted on 02 Oct 2013
In vitro, β-amyloid (Aβ) peptides form polymorphic fibrils, with molecular structures that depend on growth conditions, including various oligomeric and protofibrillar aggregates.
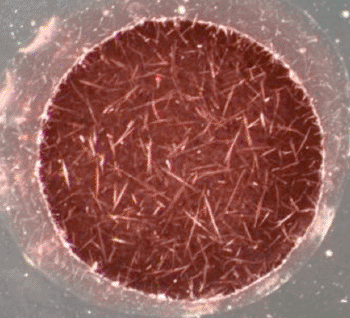
Image: Dark field scattering micrograph showing the accumulation of amyloid structures (Photo courtesy of the University of Toronto).
Scientists at the US National Institute of Health (NIH, Bethesda, MD, USA) working with colleagues from the University of Chicago (IL, USA) investigated structures of human brain-derived Aβ fibrils using seeded fibril growth from brain extract and data from solid-state nuclear magnetic resonance and electron microscopy.
They extracted β-amyloid fibril fragments from the brain tissue of two patients with different clinical histories and degrees of brain damage and then used these fragments to grow a large quantity of fibrils in a dish. They found that a single predominant 40 residue Aβ (Aβ40) fibril structure prevailed in the brain tissue of each patient, but the molecular structures were different between the two patients. A molecular structural model developed for Aβ40 fibrils from one patient reveals features that distinguish in vivo- from in vitro-produced fibrils.
Robert Tycko, PhD, a scientist at the NIH, and senior author of the study, said, “This work represents the first detailed characterization of the molecular structures of beta-amyloid fibrils that develop in the brains of patients with Alzheimer's disease. This detailed structural model may be used to guide the development of chemical compounds that bind to these fibrils with high specificity for purposes of diagnostic imaging, as well as compounds that inhibit fibril formation for purposes of prevention or therapy. This may mean that fibrils in a given patient appear first at a single site in the brain, and then spread to other locations while retaining the identical molecular structure.” The study was published on September 12, 2013, in the journal Cell.
Related Links:
US National Institute of Health
University of Chicago