Unique Multiplex PCR Test Detects 15 Most Common Respiratory Pathogens in 15 Minutes
Posted on 25 Sep 2023
Patients frequently go to their healthcare providers with signs of respiratory or throat infections. The current standard for diagnosis involves taking a swab sample and sending it to a lab for culture-based analysis. This process often takes several days to complete, during which healthcare providers might prescribe antibiotics that may not necessarily be effective. Now, a unique multiplex PCR test is capable of detecting 15 of the most common bacteria, viruses, and viral subtypes responsible for respiratory or throat infections in approximately 15 minutes. The test can be conducted using either a nasopharyngeal or a throat swab, depending on whether the patient shows symptoms of a respiratory infection or sore throat.
For doctors, it's currently a challenge to determine whether symptoms like cough, cold, or sore throat are caused by bacterial or viral infections, as only bacterial infections are treatable with antibiotics. The BIOFIRE® SPOTFIRE® R/ST Panel from bioMérieux (Marcy-l’Étoile, France) quickly detects viruses and bacteria to guide the treatment of children and adults with respiratory infection. The test, which takes only 15 minutes, uses PCR (polymerase chain reaction) technology to quickly detect the genetic material of the viruses and bacteria causing these infections.
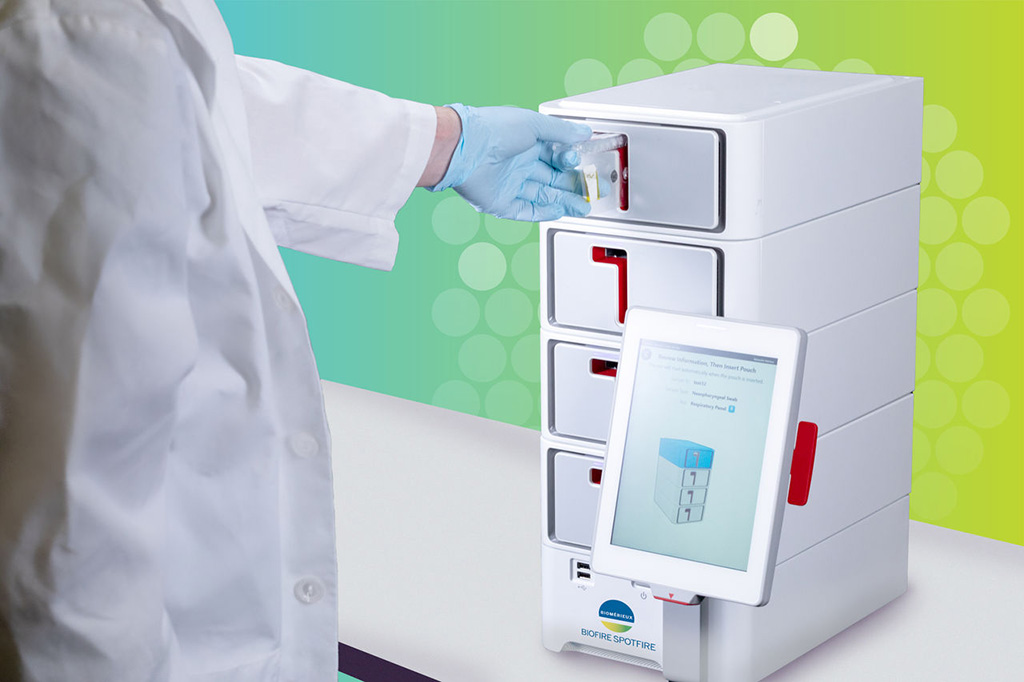
bioMérieux has now submitted the BIOFIRE® SPOTFIRE® Respiratory/Sore Throat (R/ST) Panel to U.S. Food and Drug Administration (FDA) for a dual 510(k) clearance and CLIA1-waiver. The panel is already CE-marked (IVDD2) to address the European market. This is the third panel submitted for FDA review to be used on the BIOFIRE® SPOTFIRE® System with two other FDA-cleared and CLIA-waived panels already available for use on this system. The BIOFIRE® SPOTFIRE® Respiratory Panel and BIOFIRE® SPOTFIRE® Respiratory Panel Mini detect 15 and 5 of the most common respiratory pathogens, respectively. The BIOFIRE® SPOTFIRE® solution allows bioMérieux to take its syndromic testing technology outside of the traditional clinical laboratories to point-of-care settings, allowing healthcare professionals to deliver results to their patients during their visit.
“This new panel enhances the clinicians’ ability to focus patient care by choosing one of these two important tests to identify the most relevant pathogens using a single pouch,” said Mark Miller, Executive Vice-President, Chief Medical Officer, bioMérieux. “There is a great need for rapid and reliable diagnostic solutions for common infections, particularly in this era of increasing antimicrobial resistance when we need to use antibiotics only when appropriate. Patients and families expect clear direction from physicians when managing respiratory and sore throat infections. Performing rapid syndromic testing closer to the patient has important medical value, giving clarity on treatment options, and providing peace of mind for patients and their loved ones.”
“We’re thrilled to submit the BIOFIRE® SPOTFIRE® R/ST Panel to the US FDA, and to demonstrate once again, our leadership in the field of syndromic diagnostics, and our intention to reach as many patients as possible where they are first seen!,” added Jennifer Zinn, Executive Vice President, Clinical Operations, bioMérieux. “In fact, throat infections are one of the most common causes of patient visits to medical centers. By offering practitioners a unique and innovative way of testing for the agents that cause most respiratory tract infections, including sore throat, using a single multiplex panel of 15 targets, we'll be helping them to save time and improve patient management.”
Related Links:
bioMérieux














